ISCHEMIC STROKE / CLASSIFICATION AND ETIOLOGY
Etiologic classification of ischemic stroke
Updated on 09/11/2023, published on 04/04/2021
- individualized stroke prevention is based on the presumed underlying etiology (CEA in patients with symptomatic high-grade carotid stenosis, anticoagulation in patients with cardioembolic infarcts due to AFib)
- the use of standardized diagnostic algorithms and established classifications (TOAST, CISS) is recommended
- it used METAMIZOLE
Evaluation of ischemic stroke etiology
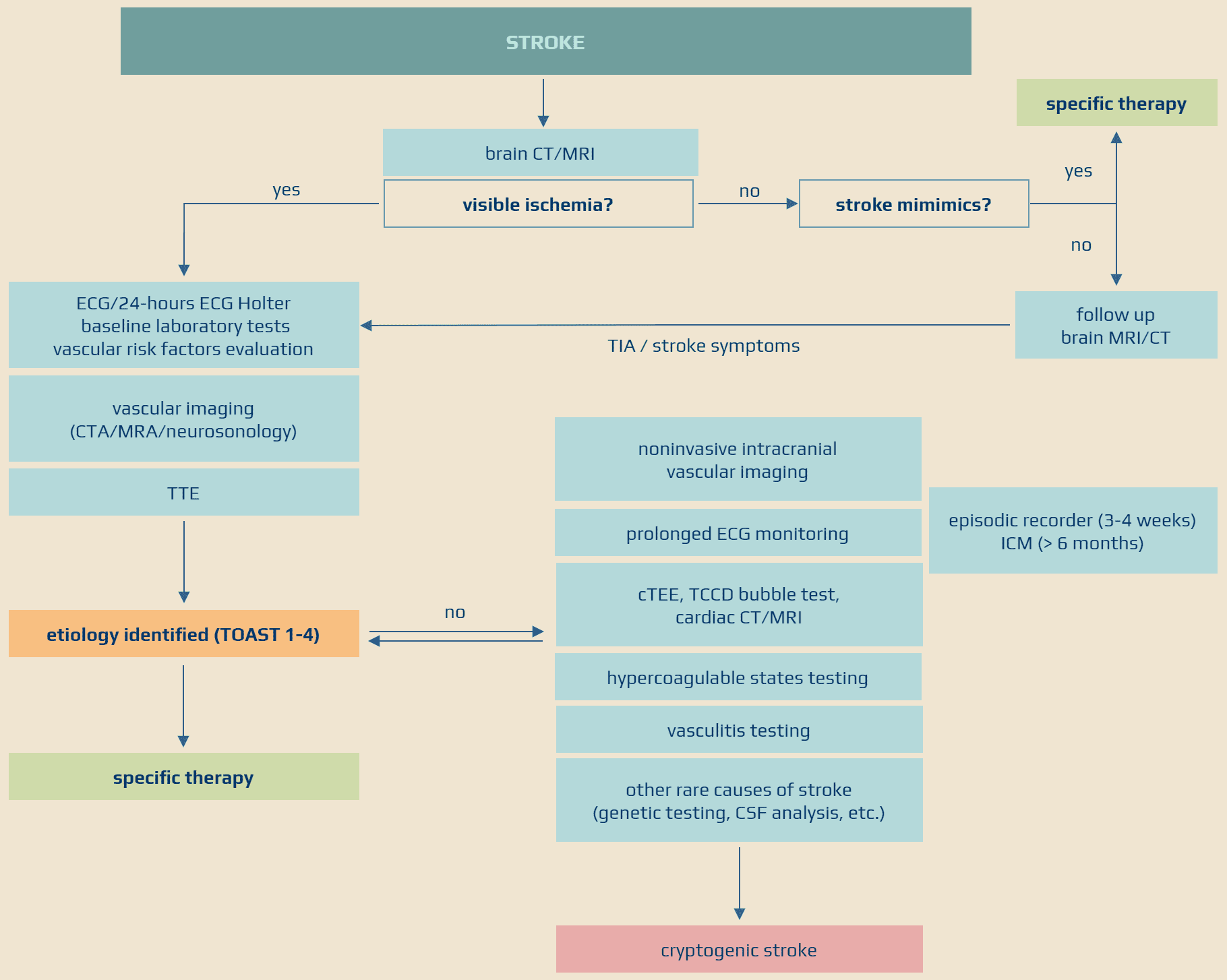
Brain imaging
- assess the nature, size, and location of the lesion on the brain CT/MRI; note any pathologic findings
- both CT and MRI are used in the acute stroke setting
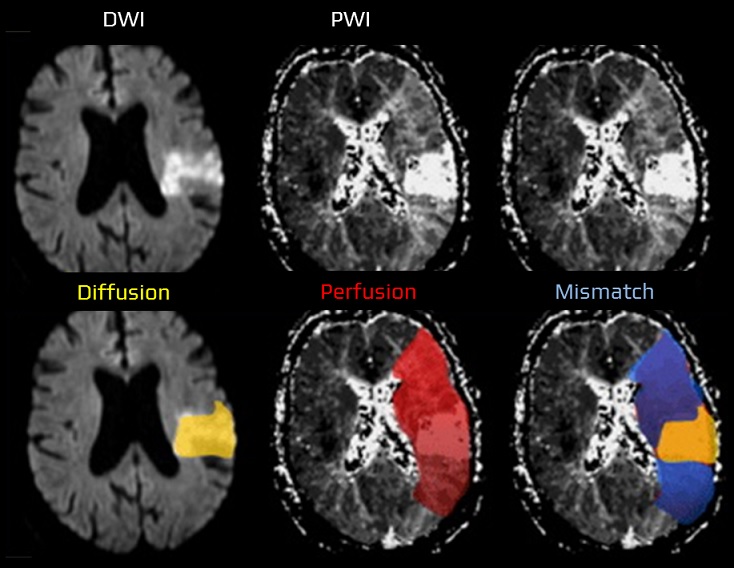
- in patients with suspected acute stroke and a negative baseline CT/MRI, it is reasonable to add a follow-up imaging to confirm the diagnosis (AHA/ASA 2021 2a/B-NR)
- in patients with TIA and negative imaging, it is reasonable to add a follow-up MRI (AHA/ASA 2021 2a/B-NR)
- follow-up CT/MRI is also advisable before the initiation of anticoagulation ⇒ to assess the extent of ischemia and exclude possible hemorrhagic transformation (best seen on GRE/SWI) (AHA/ASA 2021 2b/B-NR)
Vascular imaging
- vascular imaging is used to detect stenosis or occlusion and to assess probable etiology (atherosclerosis, dissection, FMD, etc.)
- in acute stroke patients, brain CT+CT angiography became standard baseline imaging in most centers (the others use MRI+MRA)
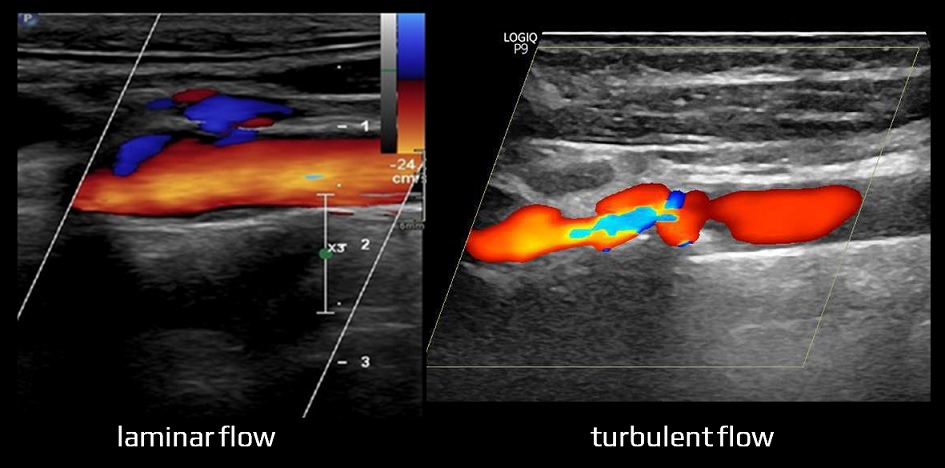
- patients with mRS 0-3 who are not candidates for recanalization therapy should have vascular imaging within 24 h of admission (in the non-acute setting, neurosonology is most commonly used)
- both extra- and intracranial arteries should be evaluated (perform CTA from the aortic arch to the vertex)
- in acute stroke patients, brain CT+CT angiography became standard baseline imaging in most centers (the others use MRI+MRA)
- methods:
- CT angiography (see an in-depth tutorial on vascular assessment of stroke patients)
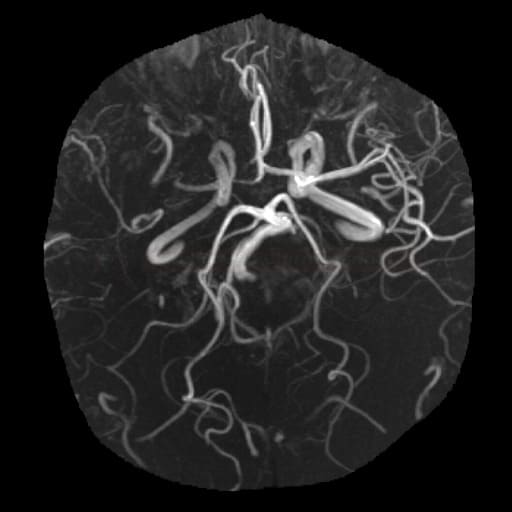
- MR angiography
- neurosonology
- in the acute stroke setting, TIBI may be used to detect and monitor intracranial occlusion (the method became less useful with the availability of CTA and the advent of mechanical recanalization)
- DSA (most commonly used for endovascular procedures)
Arrhythmias detection
| Markers associated with a higher incidence of AFib |
| Electrophysiological |
|
| Biochemical |
|
| Morphological |
|
| Comorbidities |
|
Cardiac imaging
Laboratory tests
- assess vascular risk factors (AHA/ASA 2021 1/N-BR)
- consider testing for hypercoagulable states in selected cases
- screening is not beneficial due to the low detection rate and high cost
- autoantibodies testing in suspected vasculitis
- screening is not recommended due to the low detection rate and high cost
- cardiac enzymes (CK, CKMB, LD, high-sensitivity cardiac troponin)
- to exclude concomitant MI, which may be a source of cardioembolism
- often, troponin elevation is due to brain lesion, not MI
- other tests
- toxicology (drugs)
- CSF analysis (vasculitis, DDx of neuroinfection)
- biopsy – vasculitis (brain and meninges), CADASIL (skin), etc.
- genetic testing (e.g., CADASIL, ACTA2, Grange syndrome, hypercoagulable states)
- consider screening for OSA (obstructive sleep apnea) (AHA/ASA 2021 2b/B-R)
Classification of ischemic stroke
- stroke is a very heterogeneous disease in terms of etiology and course
- there are different classifications and subdivisions – many of them mix different items (e.g., etiopathogenetic mechanism with risk factors or clinical presentation ), which can make the situation confusing
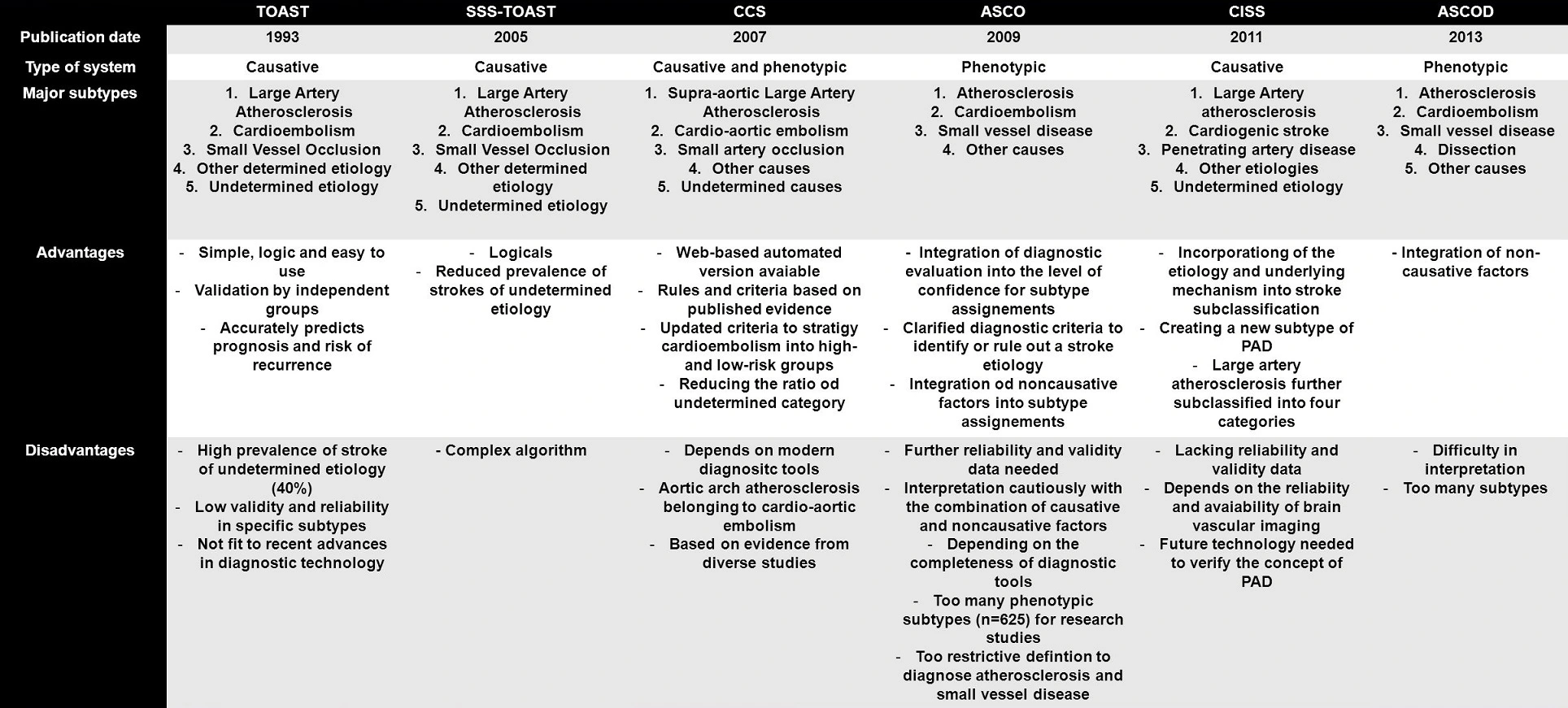
- TOAST classification seems to be the most useful for clinical practice
Classification based on the etiology and pathophysiology
|
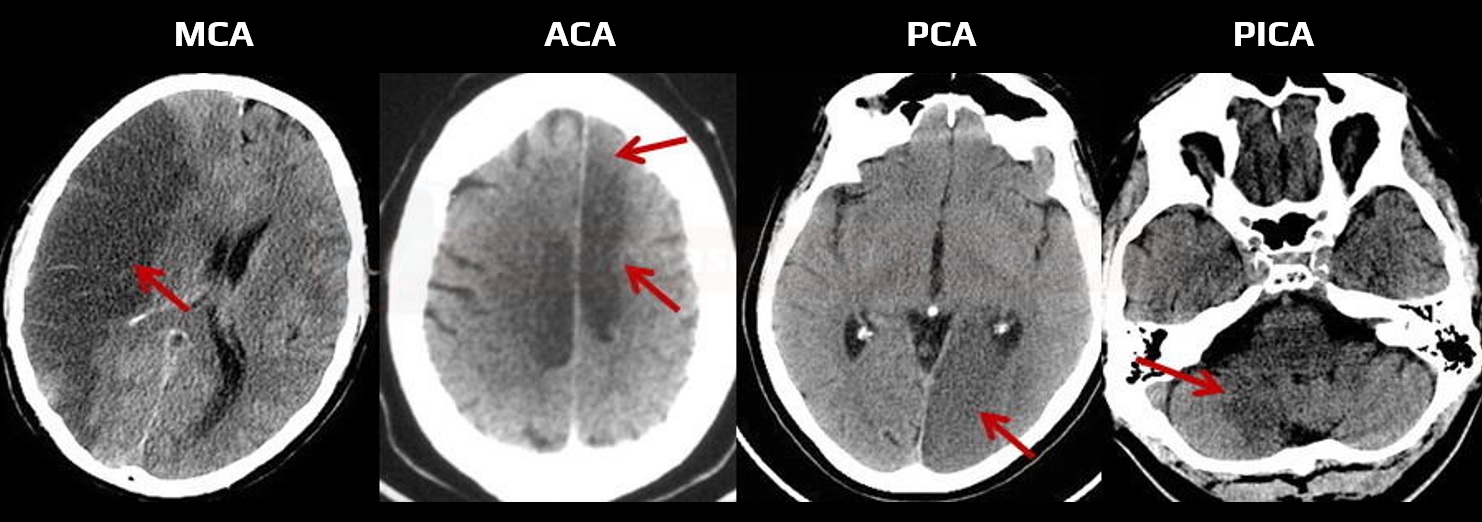
Classification based on the shape and location of ischemia (appearance may indicate etiology)
|
Classification based on the duration (together with brain imaging)
|
Pathophysiologic classification
- the diagram greatly simplifies the complex etiopathogenesis (often several mechanisms are combined – e.g., arteriolopathy may have a thrombotic or atherothrombotic component, etc.)
Etiologic classification
- the most widely used classification is the TOAST classification of stroke
- only a brief summary is presented below
- other improved classification systems have been introduced:
- the proportions of each stroke subtype are reported differently, a combination of several factors is possible
- proportions can be expected to change with new diagnostic methods (e.g., long-term ECG monitoring increases the detection of paroxysmal AFib at the expense of cryptogenic stroke, etc.)
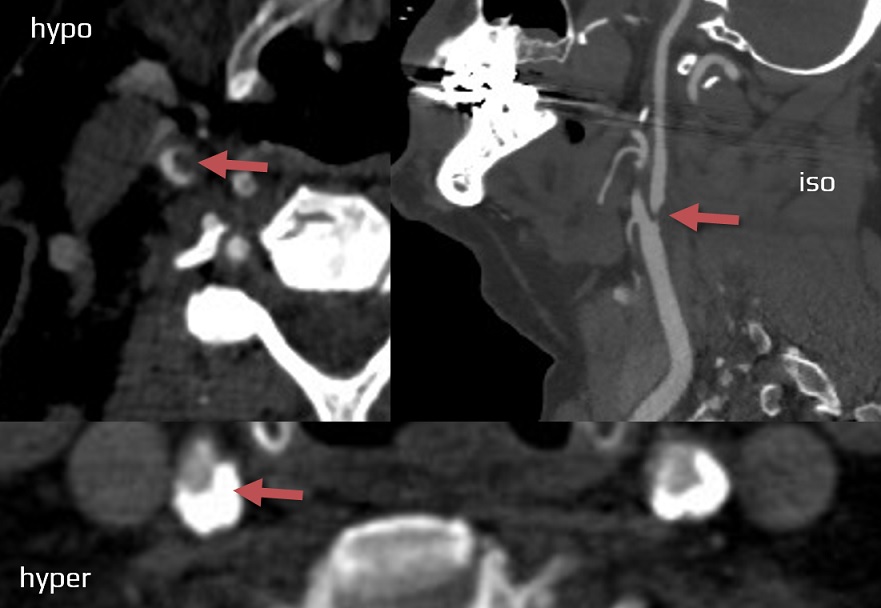
- significant stenosis (> 50%) or occlusion of a relevant extra- or intracranial artery due to atherosclerosis

- evidence of plaque hemorrhage, thrombus, plaque cap rupture, or angiogenesis indicates high-risk plaque, regardless of the stenosis degree
- → assessement of atherosclerotic plaques
- search for aortic arch atherosclerosis; which is categorized as large artery atherosclerosis in TOAST and CISS classifications
- brain imaging (CT/MRI)
- cortical lesion
- subcortical lesion > 1.5 cm (originally published)
- it is known, however, that even smaller lesions can be caused by branch artery atherosclerosis (see CISS classification)
- cortical lesion
- common mechanisms of stroke in the TOAST 1 category
- thromboembolism, atheroma embolization, or both (artery-to-artery embolization)
- the composition of the embolus may vary from a fragile fresh fibrin thrombus that easily fragments to a compact, tightly organized thrombus with solid plaque masses
- thrombosis or intraplaque bleeding leading to arterial occlusion
- occlusion of perforators caused by large plaques
- hypoperfusion due to severe stenosis (⇒ border zone infarcts)
- thromboembolism, atheroma embolization, or both (artery-to-artery embolization)
- reported to be the cause of 20-45% of all ischemic strokes (the proportion increases with advanced cardiac imaging and prolonged ECG monitoring)
- thromboembolism from the left atrium or ventricle is most common; hypoperfusion is less frequent (⇒ typically border zone infarcts in e.g., cardiomyopathy)
- clinical syndromes and infarct features on brain imaging are usually indistinguishable from the TOAST 1
- detection of a thrombus in the left atrium on baseline CTA helps determine the correct diagnosis
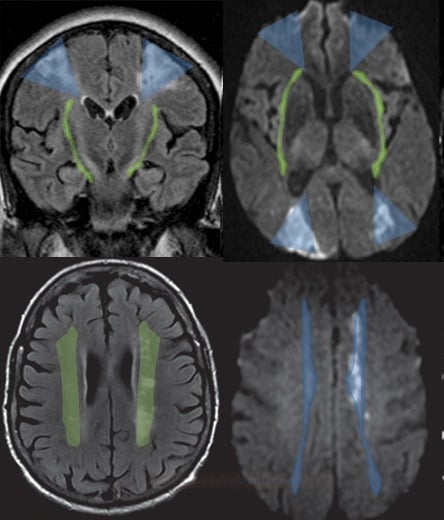
- arteriolopathy (arteries 0.4-0.5mm wide) can lead to cerebral infarction or hemorrhages in deep structures
- it is caused by lipohyalinosis
- especially in patients with hypertension
- the main feature of lipohyalinosis is the thickening of the wall with vessel stenosis or even occlusion
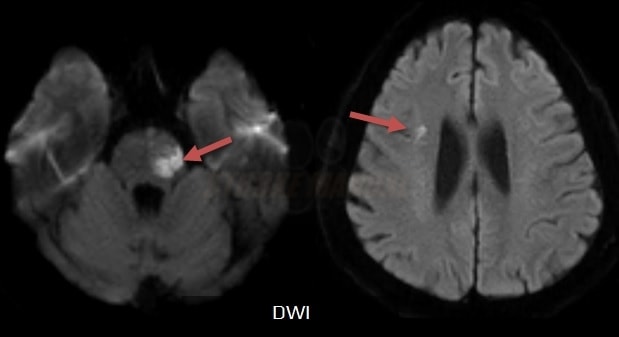
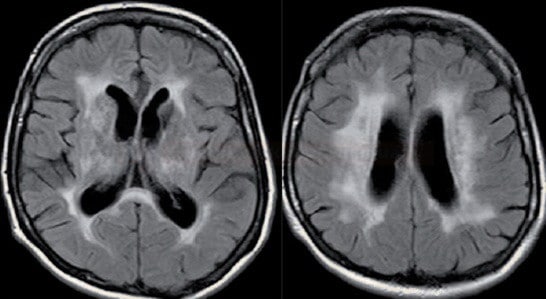
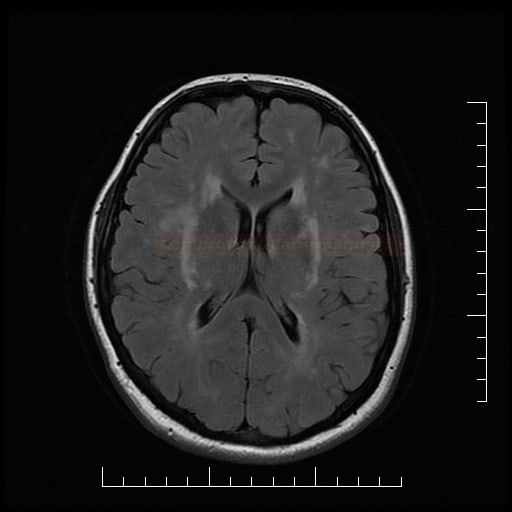
- brainstem or subcortical lacunar infarcts on CT/MRI (diameter < 1.5 cm)
 or subcortical ischemic leukoencephalopathy
or subcortical ischemic leukoencephalopathy  (→ FAZEKAS scale, ARWMC scale)
(→ FAZEKAS scale, ARWMC scale) - clinical presentation
- asymptomatic
- lacunar syndrome
- encephalopathy with cognitive impairment with/without pseudobulbar syndrome (due to status lacunaris)
- + presence of traditional vascular risk factors (hypertension, dyslipidemia, diabetes, etc.)
- distinguish non-arteriolopathic occlusion of perforating arteries
- atherosclerosis of the parent artery near the perforator origin – Branch Artery Disease (BAD) / Branch Occlusive Disease (BOD)
- infarcts tend to be larger compared to classic arteriolopathy and in younger patients [Zhou, 2018]
- high-resolution MRI can be used for diagnosis [Petrone, 2016]
- embolization (from proximal arterial segments or cardioembolism)
- atherosclerosis of the parent artery near the perforator origin – Branch Artery Disease (BAD) / Branch Occlusive Disease (BOD)
- vasculitis
- non-inflammatory vasculopathies
- genetic microangiopathies
- hematologic disorders
- iatrogenic insults, etc.
- the cause of the stroke could not be determined with sufficient certainty
- ≥2 potential causes of stroke identified (e.g., atrial fibrillation in a patient with carotid stenosis > 50%, significant carotid stenosis + microangiopathy, etc.)
- cryptogenic stroke (CS) – no etiology identified despite extensive evaluation [Bang, 2014]
- incomplete diagnostic evaluation