SUBARACHNOID HEMORRHAGE
Vasospasms after subarachnoid hemorrhage
Updated on 14/03/2024, published on 11/07/2023
- vasospasms (VSPs) in subarachnoid hemorrhage (SAH) are provoked by blood in the subarachnoid space. The breakdown products lead to changes in the arterial wall involving cytokines, inflammatory response, free radical production, and others ⇒ arterial contraction or structural changes with thickening of the vessel wall [Dorsch, 1995].
- VSPs typically appear on days 3-4, peaking on days 5-9, and may persist for up to 3-4 weeks
- when detected < 3 days after SAH, a prior episode of bleeding should be assumed
- VSPs occur in up to 70% of SAH patients (usually in the artery with the ruptured aneurysm); about 1/3-1/2 are symptomatic, leading to Delayed Ischemic Deficit (DID). DID is the leading cause of neurological impairment (focal deficits, encephalopathy) and mortality in SAH patients [Dorsch, 2002]
- patients at increased risk for developing spasms are characterized by:
- younger age
- greater volume of blood in the subarachnoid space (see Fisher grade)
- poor baseline clinical status (higher Hunt-Hess score)
- delayed initiation of treatment
- early detection and appropriate treatment of VSP can prevent or minimize the development of DID
- a clinical syndrome manifested by cognitive changes and/or focal neurological impairment lasting ≥ 1 hour and/or cerebral infarction
- present in approximately 30% of patients with SAH (Yamaki, 2019)
- multifactorial etiology
- vasospasms (VSP)
- intracranial hypertension, cerebral edema, and impaired vascular autoregulation
- presence of cortical spreading depression leading to microvascular dysfunction
- microthrombosis due to activation of the coagulation cascade and inhibition of the fibrinolysis (microemboli are detectable by TCD/TCCD)
- vasospasms (VSP)
- other causes of clinical deterioration should be excluded (rebleeding, hydrocephalus, intracranial hemorrhage, NCSE, etc.)
Vasospasms Classification
- clinical symptomatology
- asymptomatic
- symptomatic
- extent
- diffuse
- segmental
- isolated
- multifocal
- localization
- proximal
- distal
- time from the SAH onset
- early
- delayed
Clinical presentation
- worsening of headache and neck stiffness
- confusion, disorientation, decreased consciousness
- late onset of neurological impairment (hemiparesis, dysarthria/aphasia, visual disturbances)
- seizures
- fever
Vasospasms detection
TCD/TCCD
- TCD/TCCD allows repeated bedside monitoring ( → TCCD diagnosis of vasospasms)
- in vasospasms (VSP), a typical velocity increase > 50 cm/s or 25%/24h can be detected
- progression of intracranial hypertension can also be monitored → see here
CT/CTA/CTP or MRI/MRA
- non-contrast computed tomography (NCCT) excludes recurrent bleeding, obstructive hydrocephalus, or progression of cerebral edema
- CT angiography (CTA) shows the extent and severity of vasospasm
- CT perfusion (CTP) allows assessment of the risk of DID
- a prolonged transit time (TTP, MTT) is observed in the territory of the affected artery
- in severe cases, there is a decrease in regional perfusion (CBF) or blood volume (CBV) → a pattern of ischemic injury
[Binaghi, 2007]
- a prolonged transit time (TTP, MTT) is observed in the territory of the affected artery
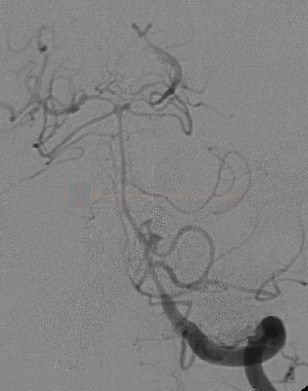
DSA
- considered the “gold standard”
- the typical finding on DSA is segmental arterial narrowing
- detection of vasospasms in peripheral, less easily visualized vessels remains a limiting factor
Management
- an individualized approach is required
- consider these factors:
- severity of the vasospasm
- presence of significant hypoperfusion in the distal circulation
- dynamics
- number and location of VSPs (focal or diffuse, single or multiple)
- signs of hyperperfusion
- signs of intracranial hypertension
- clinical status
Maintaining Cerebral Perfusion
- effect of 3H therapy (Hypertension, Hypervolemia, and Hemodilution) is not proven
- a review of controlled trials showed no beneficial effect of 3H therapy or its components on increasing cerebral blood flow (CBF) [Dankbaar, 2010]
- a review of controlled trials showed no beneficial effect of 3H therapy or its components on increasing cerebral blood flow (CBF) [Dankbaar, 2010]
- maintain normovolemia with central venous pressure (CVP) at 10-12 cm H2O (mm Hg) or pulmonary artery wedge pressure (PAWP) at 14-20 mmHg
- maintain hematocrit at 30-35% to optimize blood viscosity
- systolic blood pressure (SBP) should be maintained at ≤ 130-140 mmHg preoperatively and 150-175 mmHg postoperatively
- administer inotropes and vasopressors if mean arterial pressure (MAP) is < 90 mm Hg
Drug therapy
- nimodipine and cilostazol are likely the most effective treatments for preventing morbidity and mortality (Dayyani, 2022)
- clazosentan, nicardipine, and magnesium have beneficial effects on delayed cerebral ischemia and vasospasm, but they were not found to reduce mortality/disability (Dayyani, 2022)
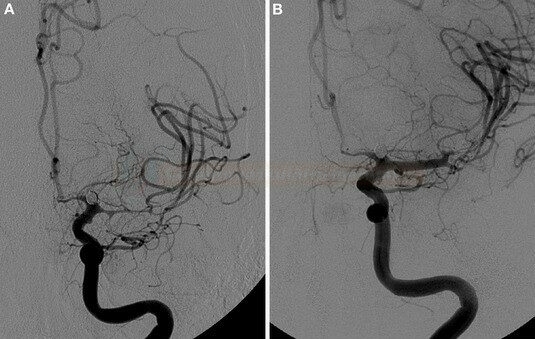
Percutaneous angioplasty (PTA), local application of vasodilators
- PTA should only be considered for symptomatic vasospasms after failure of conventional therapy
- PTA results in mechanical disruption of the spasm; the effect is long-lasting
- alteration of myocyte structure
- disruption of collagen in the vessel wall
- can be performed in proximal VSPs (siphon, M1, A1, V4, AB, and P1); the procedure is associated with frequent complications (vessel rupture, dissection, or occlusion)
- the best clinical effect can be expected in patients with an initial Hunt-Hess score of 1-3 who are treated within 2 hours after the onset of clinical symptoms of vasospasm (improvement in up to 80%) [Zubkov, 1994]
- CT perfusion may be used for appropriate patient selection
- procedure should be performed in patients with large penumbra and a small core
- in the presence of a large core, the effect cannot be expected, and the risk of hemorrhagic transformation is high
- PTA in the presence of an untreated aneurysm increases the risk of rebleeding; it is advisable to treat the aneurysm first
- if the aneurysm cannot be treated because of spasms, local vasodilators should be used instead of PTA
- angioplasty may be combined with local application of vasodilators; dosing is not standardized and mostly based on case reports and small cohorts
- appropriate for diffuse or distal involvement
- short-term effect compared with angioplasty
- papaverine is no longer used (as it induces systemic hypotension and precipitates with heparin (may cause microembolization)
(Dilceren / Nimotop) usually 1 mL/0.2 mg
- IV: 1-2 mg/h (5-10mL/h) by continuous infusion + 10 ml/h NS concomitantly
- AEs: hypotension, tachycardia
- combine with vasopressors in case of hypotension
- parenteral administration is not more effective than oral administration in preventing vasospasm
- infusion pumps with polyethylene tubing and needles with polyethylene handles (or all-metal) must be used, infusion solution is light sensitive, must not be used in direct sunlight
- IA: 50 mL (10mg) + 50mL NS (to get 10% solution; 10mL containing 1mg) … IA infusion 1mL/min (0.1mg /min)
-
0.5-2 mg into a single artery, total max dose 5 mg [Kim,2009]
-
(Verapamil / Isoptin / Lekoptin) usually 1mL/2.5 mg
- IA: bolus 1-3 mg (or 44 μg/kg) locally [Feng,2002]
- AEs: hypotension, bradycardia
(Perlinganit)
- IA: 0.5-2 mg as a slow bolus
(Asicord / Primacor)
- IA: 1mg/ml – 0.25 mg/min – infused for 30 minutes
- regression of vasospasm with combined IA and subsequent IV administration has been reported [Arakawa, 2001] [Fraticelli, 2008]
- IV administration alone seems to be effective [Crespy, 2019]
(Magnesium sulfate) usually 1mL/40 mg or 1mL/80mg
- IA: 10mL (1g) + 28 mL NS …. 0.25-1 g per artery [Shah, 2009]
- may be used in combination with nicardipine (2.5-20.0 mg/h for 30-60 min)
- magnesium is believed to inhibit cerebral vasospasm by causing smooth muscle relaxation and vasodilation by mechanisms similar to the calcium channel antagonists
- IV: a large phase III study showed no significant benefit in cerebral vasospasm prevention or improved favorable outcomes when administered via IV infusion (Wong, 2010)
(Papaverine hydrochloride) 1mL/30mg
- IA: 300mg (10 mL) + 100mL of NS (solution concentration is < 3mg/mL) …. IA infusion 3mL/min for 30 minutes or 1.3 mL/min for 60 minutes [Kassel, 1992][Clouston, 1995]
- usually not used due to AEs
Thrombolytic therapy
- meta-analysis suggests a clinically relevant beneficial effect of subarachnoid clot lysis achieved by intracisternal tPA injections (Amin-Hanjani, 2004]
- ARR of 14.4% for delayed ischemic neurological deficit
- therapy is associated with an increased risk of intracranial bleeding
- clinical trials are needed to determine the safety and efficacy of this approach