GENERAL THERAPY
Intracranial hypertension
Updated on 10/05/2024, published on 04/12/2023
Definition
- intracranial hypertension is defined as an increase in intracranial pressure (ICP) above the normal range (commonly used threshold is > 20 mmHg or 27 cm H2O)
- the normal ICP range for adults in a horizontal position is ~ 7-15 mmHg; 1 mm Hg = 1.36 cm H2O
- IC hypertension occurs due to an increase in the volume of one or more of the main intracranial compartments (brain, CSF, blood vessels) due to the failure of the compensatory mechanism
Pathophysiology
- the neurocranium forms a closed and rigid shell; normally, there are 3 incompressible environments in equilibrium (Monro-Kellie doctrine) :
- brain (80-90%)
- cerebrospinal fluid (5-10%)
- blood volume (5-10%)
- normal intracranial pressure (ICP) is ~ 5−15 mm (increasing transiently with coughing, physical exertion, in Trendelenburg position) [Rangel-Castilo, 2008]
- ICP 16−20 mm Hg: mild hypertension
- ICP 21−40 mm Hg: moderate hypertension
- ICP 41−60 mm Hg: severe intracranial hypertension
- ICP > 60 mm Hg: critical hypertension
- normal cerebral perfusion pressure (CPP) = 70-100 mmHg
- safe CPP is > 50 mmHg
- CPP 30- 50 mm Hg leads to reversible functional impairment
- CPP < 30mm Hg may result in irreversible changes
- an increase in the volume of one compartment must be compensated by a decrease in the volume of other compartments (initial compensation)
- after the exhaustion of compensatory mechanisms (critical volume), ICP starts to increase, followed by a decrease in cerebral blood flow (↓CPP)
Cerebrospinal fluid compartment
- CSF is mainly produced by the choroid plexus in the brain’s ventricles, with an hourly production rate of ~ 10-25 mL
- the total volume of CSF is ~ 90-150 mL
- CSF circulates through the ventricles, over the surface of the brain and spinal cord, and through the subarachnoid space
- it is absorbed into the bloodstream via Pacchioni granulations
- granulations are predominantly located along the superior sagittal sinus but can also be found in other dural sinuses
- CSF flows through these granulations into the dural venous sinuses, thereby maintaining CSF homeostasis
- permeability pressure of CSF is ~ 5mmHg
- if the ICP rises, the CSF is resorbed faster, leading to a decrease in its volume (see below)
Vascular compartment
- arterial portion – high-pressure system with active autoregulation
- venous portion – low-pressure, capacitive system, relatively that is relatively passive
- both parts can change their volume; the venous portion responds to an increase in ICP by reducing the volume of the compressible parts
- autoregulation may be impaired in the affected area
- vasoparalysis (with vasodilatation) increases vascular volume and leads to the progression of vasogenic edema, thereby increasing ICP
- autoregulation is also impaired in advanced age or due to significant proximal arterial stenosis (where peripheral resistance increases and cerebrovascular reserve decreases)
- vasoparalysis (with vasodilatation) increases vascular volume and leads to the progression of vasogenic edema, thereby increasing ICP
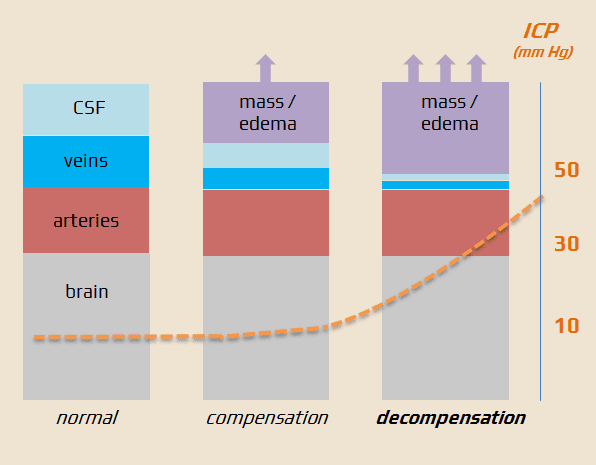
Compliance
- the cranial compartment is rigid with a fixed total volume of brain tissue, blood, and cerebrospinal fluid (CSF). According to the Monro-Kellie rule, any increase in one of these components must be compensated by a decrease in the others to maintain stable intracranial pressure (ICP)
- compliance = capacity to accommodate changes in volume without a significant increase in ICP
- reduced compliance means that a smaller increase in intracranial volume can cause a significant rise in ICP
- factors affecting compliance
- age (compliance decreases with age)
- pathology (e.g., edema, hemorrhage)
- physiological changes (e.g., arterial and venous pressure variations)
- compliance curve = curve, where an initial increase in intracranial volume results in minimal changes in ICP due to compensatory mechanisms
- once these mechanisms are exhausted, further volume increase leads to a sharp and rapid rise in ICP
- a correlate of exhausted compliance is the presence of herniation signs on neuroimaging
| Intracranial hypertension can occur due to an increase in the volume of any of the three components mentioned |
|
| Cerebrospinal fluid (CSF) |
→ all resulting in fluid accumulation and increased pressure |
| Blood |
|
| Brain tissue |
|
| Cerebral edema is classified into three main types based on the underlying pathophysiological mechanisms: |
|
| 1. Extracellular | |
|
1.1 Vasogenic
1.2 Hydrocephalic (interstitial)
|
|
|
2. Intracellular (cytotoxic)
|
|
|
Clinical presentation
- headache
- typically, the earliest and most common symptom
- gradually progressing
- the pain increases with pressure on the jugular veins or during the Valsalva maneuver
- the supine position increases the pain, while the vertical position decreases it
- typically, the earliest and most common symptom
- nausea, vomiting
- “central” vomiting (sudden, projectile)
- classic vomiting accompanied by nausea
- “central” vomiting (sudden, projectile)
- vertigo
- most commonly experienced as a feeling of imbalance; lesions in the posterior fossa may provoke rotational vertigo
- blood pressure
- initially, BP rises as a regulatory mechanism to maintain cerebral perfusion pressure (CPP)
- with progressive intracranial hypertension, this mechanism fails, leading to a drop in BP
- secondary bradycardia (due to vagal irritation) may also occur
- initially, BP rises as a regulatory mechanism to maintain cerebral perfusion pressure (CPP)
- tachycardia
- sudden change from bradycardia to tachycardia may indicate vagal paralysis ⇒ Cushing’s triad
- sudden change from bradycardia to tachycardia may indicate vagal paralysis ⇒ Cushing’s triad
- respiratory disorders
- cranial nerve palsies
- particularly involving CN VI (abducens nerve), leading to double vision
- coma and unilateral non-reactive mydriasis are typical signs of uncal herniation
- oliguria
- caused by diencephalon compression
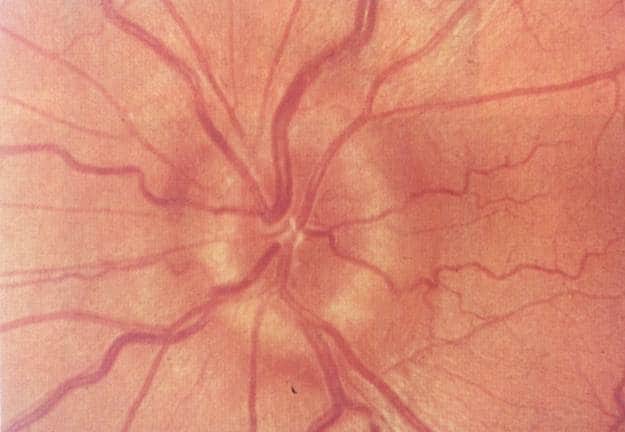
- papilledema
- altered mental status
- from drowsiness to confusion; in severe cases, sopor or coma occur
- from drowsiness to confusion; in severe cases, sopor or coma occur
- brain herniation symptoms → syndrome of rostrocaudal deterioration
- progressive consciousness disorder
- progressive paresis, decerebrate or decorticate rigidity
- oculomotor disturbances
- uncal herniation leads to ipsilateral mydriasis due to direct mesencephalon compression
- contralateral mydriasis is caused by the shifting of the mesencephalon against the contralateral tentorium
- Cushing triad (hypertension + bradycardia + respiratory disturbances) indicates brainstem compression
- the triad reflects the body’s physiological response to severe intracranial hypertension, attempting to maintain CPP; triad is considered a neurosurgical emergency
Diagnostic evaluation
Medical history and clinical evaluation
- symptoms and signs are discussed above
- neurological findings depend on the etiology of intracranial hypertension
- careful monitoring of consciousness level is crucial (using GCS, Beneš-Drábek scale)
- in patients with impaired consciousness, brainstem reflexes monitoring is essential to assess the development of rostrocaudal deterioration syndrome → see chapter Disorders of consciousness
Ophthalmologic examination
- the head of the optic nerve (CN II) is swollen, with blurred borders
- protrusion can be measured in diopters
- arteries are narrowed, veins are widened and filled with stagnant blood
- small punctate hemorrhages may be observed
- in the initial phase of papilledema, vision is not impaired; prolonged congestion leads to optic atrophy with visual impairment
Neuroimaging
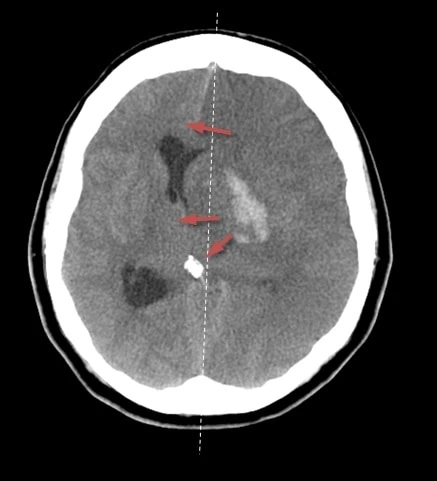
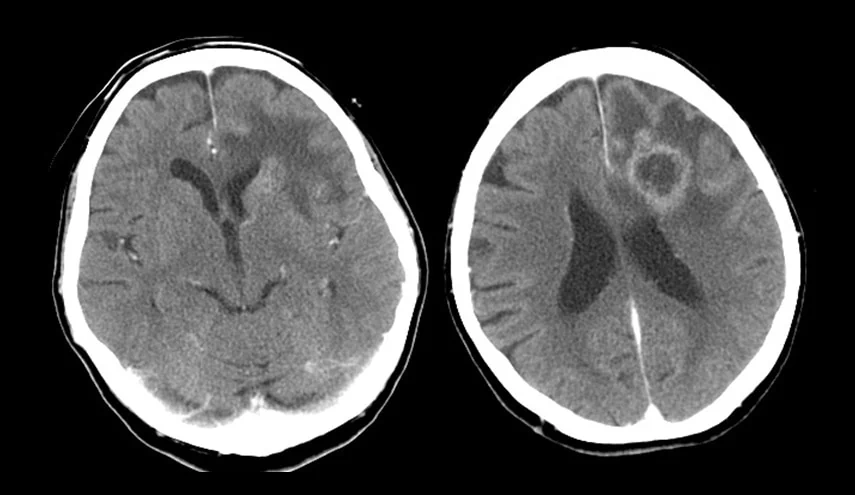
- monitoring of parenchymal lesions (e.g., measurement of hematoma size, assessment of hemorrhagic transformation of contusion or ischemia, development of edema)
- symptoms indicative of compliance exhaustion
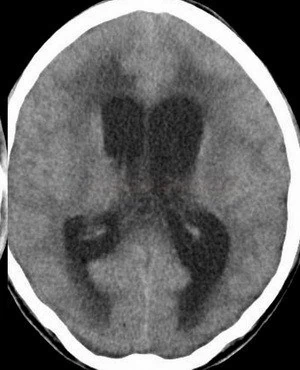
- narrowing of the ventricular system (except for complications causing hydrocephalus)
- disappearance of subarachnoid spaces
- compression of the lateral ventricles
- compression of the basal cisterns
- midline structures shift (watch pineal calcification)
- signs of non-communicating hydrocephalus include dilatation of the ventricles above the obstruction with transependymal fluid transfer (hypodense periventricular rim on CT, hyperintense areas on T2-weighted MRI)
Neurosonology
Intracranial pressure monitoring
- allows verification and quantification of intracranial hypertension
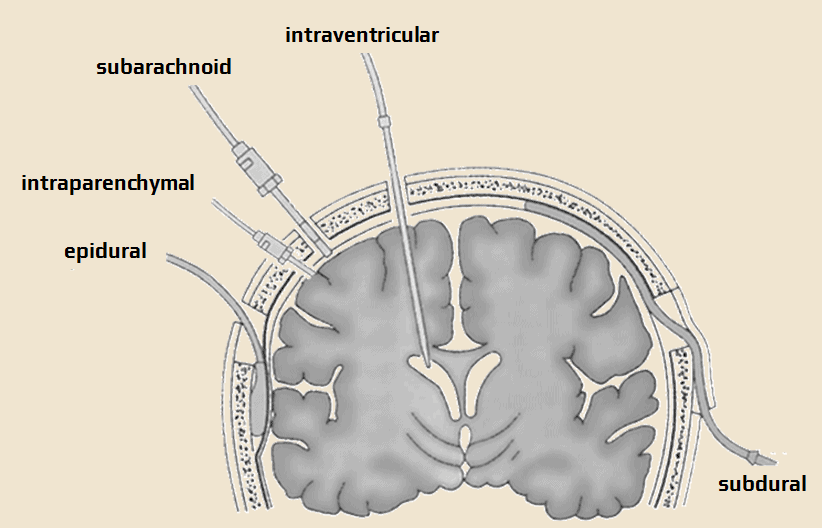
- IC pressure measurement:
- invasive using different types of sensors:
- intraparenchymal (in parenchymal lesions)
- intraventricular (used when CSF of blood drainage from the lateral ventricles is presumed)
- subdural or epidural
- non-invasive (using transcranial Doppler)
- invasive using different types of sensors:
- indications unclear except for TBI
- essential in traumatic lesions with GCS < 8
- indication is less clear in ICH, although it’s probably useful in extensive intraventricular bleeding
- in acute hydrocephalus, external drainage is also a therapeutic procedure
- the sensor should be used for a maximum of 5-7 days, after which the risk of infection increases and the accuracy of the measurement decreases
- indications for full therapy of intracranial hypertension include persistent ICP above 15-20 mmHg or CPP < 50 mm Hg
Monitoring oxyhemoglobin (SjO2) saturation in the jugular bulb
- monitoring arteriovenous (A-V) difference in SjO2 is a technique used to assess the balance between cerebral oxygen supply and demand; it provides insight into cerebral hemodynamics and metabolism.
- a catheter is placed in the jugular bulb to measure venous O2 saturation
- by comparing SjO2 with arterial oxygen saturation (SaO2), clinicians can infer the brain’s oxygen utilization
- normal range 55-80% (difference 20-30%)
- a decrease in SjO2 < 50% with normal arterial oxygen saturation indicates either ↓CBF (e.g., due to increased cerebrovascular resistance) or increased cerebral O2 consumption (↑CMRO2)
- an increase in SjO2 > 85% (lower AV difference) indicates either hyperemia with ↑CBF or reduced cerebral metabolic demand (↓CMRO2 in neuronal death)
- normal range 55-80% (difference 20-30%)
- it is necessary to take into account body temperature and the influence of medication during the evaluation
- the technique reflects global cerebral oxygenation and may not accurately represent regional variations, especially in focal brain injuries
- useful in traumatic brain injury; its efficacy in stroke is unknown