ISCHEMIC STROKE / COMPLICATIONS
Malignant Cerebral Infarction
David Goldemund M.D.
Updated on 27/07/2024, published on 20/06/2023
Definition
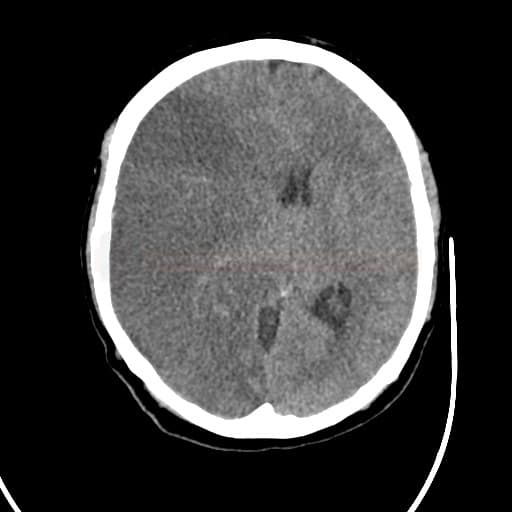
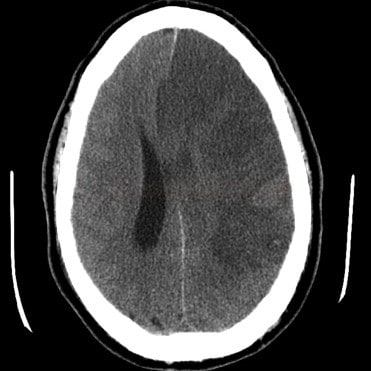
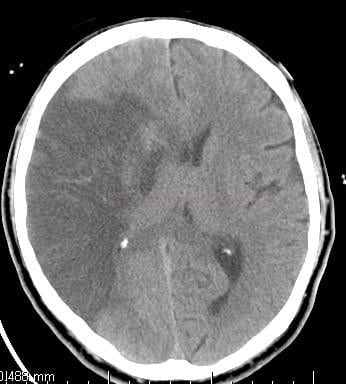
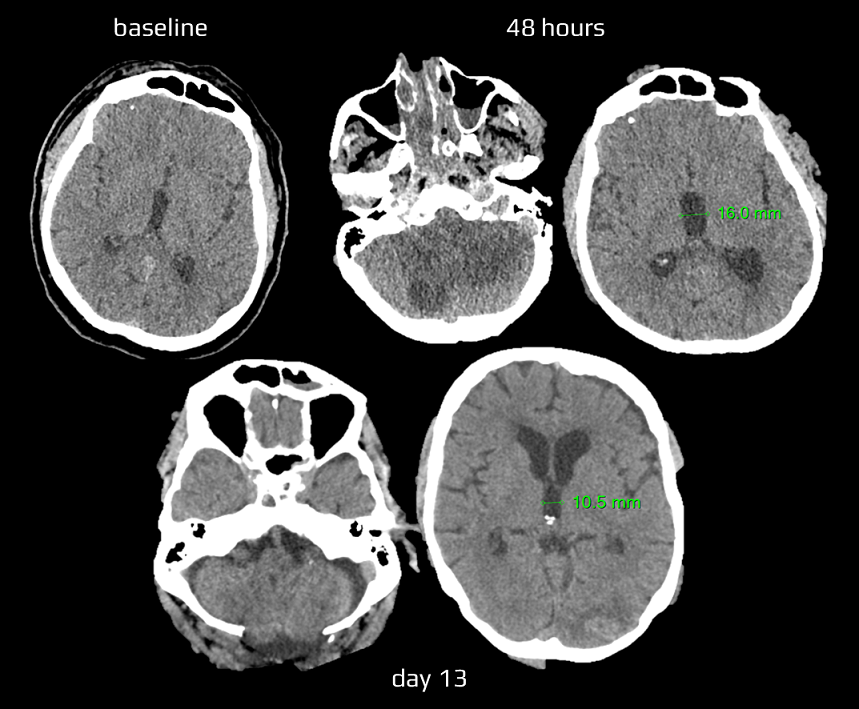
- malignant cerebral infarction = extensive infarction with significant space-occupying brain edema causing intracranial hypertension
- typically occurs in young patients with large vessel occlusion (ICA and MCA), posterior circulation strokes (mainly cerebellar infarcts), and unsuccessful revascularization (Hawkes, 2024)
- traditionally associated with a high mortality rate (up to 80%) [Hacke, 1996]
- it is characterized by the development of edema within 24 hours, clinical deterioration usually in < 72 hours, sometimes later (e.g., in the case of collateral circulation failure and infarct development in the penumbra)
- randomized controlled trials (RCTs) have shown that early decompressive craniectomy reduces mortality and improves outcome in younger patients
Predictors of malignant infarction
|
Clinical predictors
|
Radiological predictors
|
|
|
Clinical features
- the initial severe neurological deficit with hemiplegia, eye and head deviation, high NIHSS score, nausea, and vomiting
- posterior circulation infarction is associated with altered consciousness, oculomotor dysfunction, altered brainstem reflexes, ataxia, and dysmetria
- progressive impairment of consciousness and conus symptoms in supratentorial lesions are due to rostrocaudal (craniocaudal) deterioration
- Cushing’s triad is present in advanced stages of intracranial hypertension
- cardiovascular lability
- cerebellar infarcts increase the risk of brainstem compression and hydrocephalus
Diagnostic evaluation
Imaging methods
- look for expansive behavior of ischemia and a midline shift on CT
- repeat brain CT within the first 48 hours in high-risk patients [AHA/ASA 2014 I/C]
- use MRI for early detection of large DWI lesions
- prediction of fulminant course within 6h: DWI lesion > 80-89 mL
- prediction of fulminant course after 14h: DWI lesion > 145 mL
Blood tests
- tests should exclude extracerebral causes of clinical deterioration
- a basic metabolic panel with hepatic enzymes
- minerals, including phosphate
- coagulation and blood count
- toxicology and arterial blood gas analysis (ABG, also known as ASTRUP) should be considered in the presence of impaired consciousness not fully explained by structural changes on CT
Other methods
- EEG excludes nonconvulsive status epilepticus (unless NCCT correlates with the severe clinical condition)
- TCD / TCCD can be used to monitor blood flow
- occlusion detection (→ TIBI classification)
- signs of intracranial hypertension
- ICP monitoring
Differencial diagnosis
| Content available only for logged-in subscribers (registration will be available soon) |
Management
Medical therapy |
- routine intensive care for acute stroke patients → see here
- consider transport to a hospital capable of performing acute decompressive craniectomy
- osmotherapy
- should be initiated only in patients with developing edema; prophylactic administration of osmotherapy is not recommended
- Mannitol / NaCl 10% (AHA/ASA 2019 IIa/C-LD)
- 0.25–0.5 g/kg (usually 20–30 g) IV over 20 min every 6 h to a maximum daily dose of 2 g/kg
- mannitol is thought to decrease ICP by decreasing overall brain water content and CSF volume and by reducing blood volume by vasoconstriction; it may also improve cerebral perfusion by decreasing viscosity or by altering red blood cell rheology
- Furosemide 40 mg IV bolus is sometimes used as an adjunct therapy in patients whose neurological condition is rapidly deteriorating
- there are no data on the benefit of hypothermia, barbiturates, and corticosteroids in stroke patients, and they are therefore not recommended
- RCTs have shown no benefit of corticosteroids in patients with acute ischemic stroke on death, neurological impairment, or functional outcome
- hyperventilation in mechanically ventilated patients
- PCO2 reduction by 5–10 mmHg can lower ICP by 25–30%
- however, vasoconstriction might compromise brain perfusion and aggravate ischemia; it should be used as a temporary measure whilst preparing to intervene definitively to control ICP
- the effect of conservative treatment in malignant ischemia is usually insufficient and not clearly demonstrated
→ Therapy of intracranial hypertension
Surgery |
- preferred in younger patients; considered very carefully in older patients
- don’t wait for the effect of hyperosmolar therapy with developing malignant ischemia (as it has only minimal effect)
- benefit of hemicraniectomy is greatest when it is performed within 48 hours of symptom onset
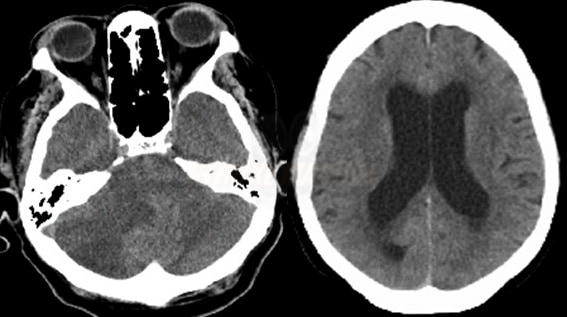
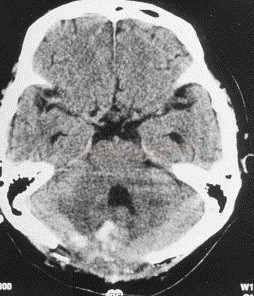
Cerebellar infarction
- alert and clinically stable patients are usually treated conservatively and monitored closely (although some authors recommend early or preventive intervention)
- surgery is required in the case of extensive mass effect and clinical signs of deterioration
- suboccipital decompressive craniectomy +/- resection of necrotic tissue for expansive cerebral ischemia may be a life-saving procedure (AHA/ASA 2019 I/B-NR)
- ischemia leads to brainstem and aqueduct compression, so craniectomy can also relieve obstructive hydrocephalus
- it can be combined with the ventricular drainage
- ventriculostomy for obstructive hydrocephalus due to expansive cerebellar infarction (with or without craniectomy) (AHA/ASA 2019 I/C-LD)
- suboccipital decompressive craniectomy +/- resection of necrotic tissue for expansive cerebral ischemia may be a life-saving procedure (AHA/ASA 2019 I/B-NR)
- mass effect imaging criteria:
- compression of the 4th ventricle
- obstructive hydrocephalus
- basal cisterns/brainstem compression
- upward herniation of the superior vermis cerebelli through the tentorial notch
- downward herniation of the cerebellar tonsils through the foramen magnum
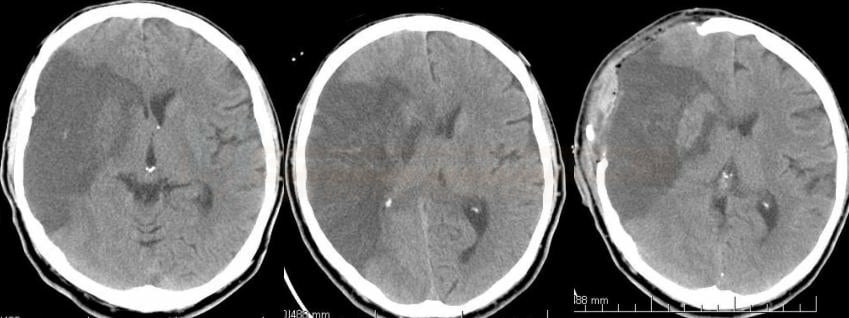
Supratentorial stroke (usually MCA territory)
- decompressive hemicraniectomy relieves pressure from the edematous tissue on adjacent tissue ⇒ ↓ ICP and ↑ CPP
- potentially life-saving procedure (see below for additional indication criteria)
- age < 60 years – a meta-analysis of the DESTINY, HAMLET, and DECIMAL trials demonstrated that decompressive craniectomy within 48 hours in patients aged <60 years resulted in reduced mortality and improved outcome (AHA/ASA 2019 IIa/A)
- age > 60 years – reduced mortality alone has been demonstrated in smaller studies [Won Yu, 2012] and randomized DESTINY II trial
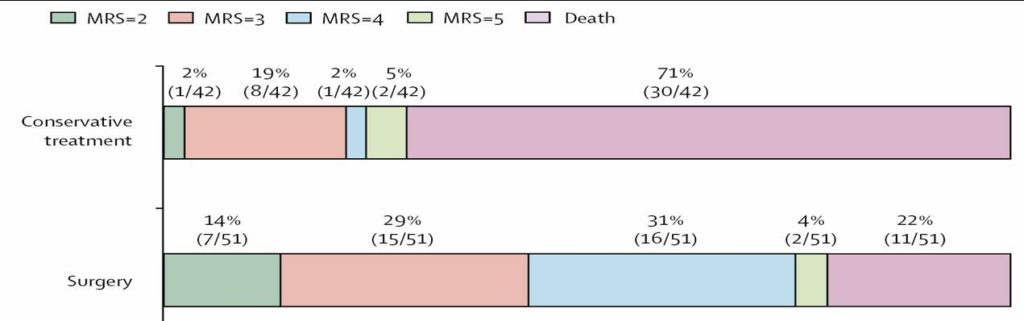
- n = 112 (surgery 49 vs. control 63), age> 61 years
- mRS 0-4 39% (surgery) vs. 17% (control), ARR 22, NNT = 5 !!
- significant reduction of mortality (50%); compared to trials with patients < 60 years, there is a significant increase of survivors with very severe deficits and only a minimum of patients with mRS 3 (none 0-2) ⇒ questionable cost-effectiveness
- there are no indications that surgery should not be considered in patients with dominant-hemisphere infarction
- craniectomy diameter of at least 12 cm
- 14-15 cm anteroposteriorly
- 0-12 cm from the base to the vertex
- simultaneous resection of infarcted tissue is usually not recommended
Indication criteria for decompressive craniectomy (used in the RCTs)
- age 18 – 60 years (younger patients have a better prognosis; at age > 60 years, mainly a reduction in mortality can be expected)
- NIHSS > 15
- somnolence-sopor
- ischemia > ½ of the MCA territory according to CT (with or without concurrent ipsilateral infarct in ACA or PCA territory)
- infarct volume > 145 cm3 on DWI
- < 45 h (surgery completed in < 48 h) from symptom onset
Exclusion criteria
- non-reactive, dilated pupils
- pre-morbid mRS ≥ 2
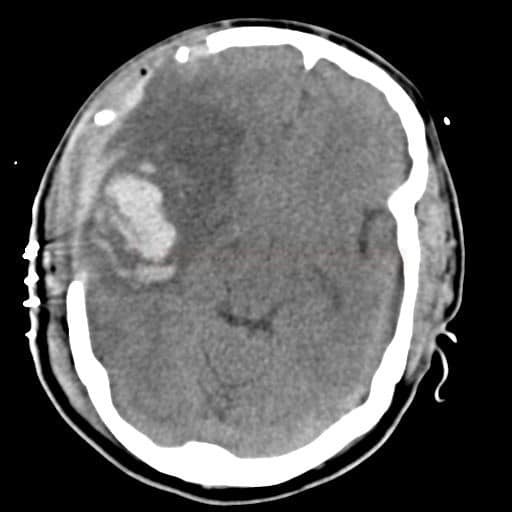
- extensive hemorrhagic component (type PH2)
- expected survival <3 years due to severe comorbidities
- coagulopathy
- contraindications to general anesthesia (GA)
Clinical trials
| Primary and secondary outcome | craniectomy | control |
| mRS 0-4 / 1 year | 75% | 25% |
| mRS 0-3 / 1 year | 43% | 21% |
| survival | 78% | 29% |
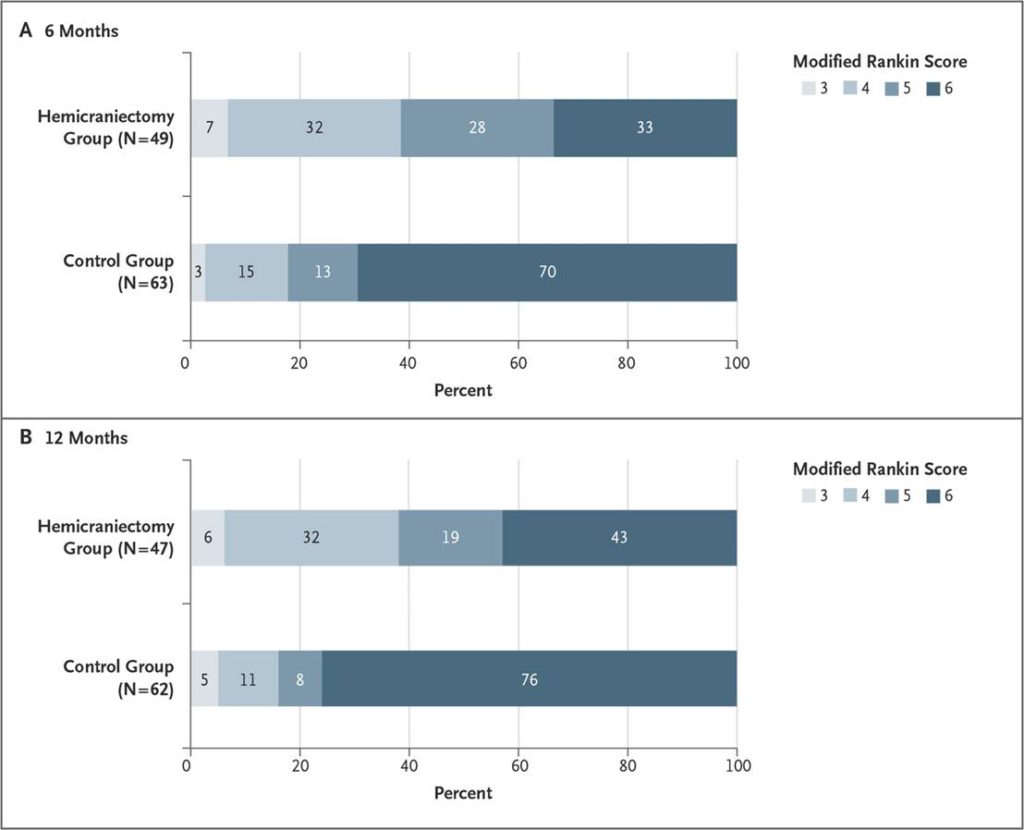
DESTINY II [Jüttler, 2014]
- total n= 112
- decompressive craniectomy, n = 49
- control group, n = 63
- > 61 years of age
- mRS 0-4 39% (craniectomy) vs 17% (control), ARR 22, NNT = 5
- significant reduction in mortality, but only a small number of patients with mRS ≤ 3 !!!!
| mRS | Decompressive craniectomy (%) | Control (%) |
| 3 | 6 | 4 |
| 4 | 33 | 14 |
| 5 | 26 | 12 |
| 6 | 35 | 70 |