ADD-ONS
Cerebral hyperperfusion syndrome (CHS)
Updated on 16/08/2024, published on 04/02/2023
-
Cerebral Hyperperfusion Syndrome (CHS) is a clinical condition characterized by a significant increase in cerebral blood flow that exceeds the autoregulatory capacity of the brain, leading to a spectrum of neurological symptoms
-
CHS can occur within hours to days after a revascularization procedure, such as carotid endarterectomy (CEA) or carotid artery stenting (CAS)
-
the peak incidence of CHS and hemorrhage after CEA is 5-7 days, while it is 12-48 hours after CAS [Ogasawara, 2007]
- a longer delay (days to weeks) has also been reported
- CHS after aortic stenosis surgery may result in bilateral lesions.
-
- diagnostic criteria include new-onset symptoms (headache, neurological deficit, and seizures) + evidence of hyperperfusion detected by TCD, MRI or SPECT
- incidence 1-14% (usually ∼ 7%)
- CEA and CAS pose similar risks (Galyfos, 2017)
- incidence seems to be higher in moyamoya surgery (Hayashi, 2012)
- the cornerstone of prevention is strict perioperative blood pressure (BP) control (for at least 14-21 days)
- TCD should be available to identify patients with hypoperfusion
Is hyperperfusion always present in symptomatic patients?
- hyperperfusion is seen in most cases of CHS, but symptoms can also occur in patients with only moderately increased CBF (30–50% above baseline) following CEA (these cases should referred to as reperfusion injury)
Procedures associated with CHS
- carotid endarterectomy
- carotid and vertebral artery stenting
- carotid and vertebral angioplasties
- extra-cranial intracranial bypass
- clipping of internal carotid artery aneurysm
- subclavian angioplasty
- revascularization in moyamoya syndrome
- repair of severe aortic valve stenosis
Pathophysiology
- the terms hyperperfusion (excessive flow/hyperemia) and reperfusion (flow normalization) are often used synonymously because both can cause cerebral injury with similar clinical presentation
- CHS results from a failure of cerebral blood flow autoregulation, leading to hyperemia
- long-term hypoperfusion, seen in conditions like high-grade stenosis, is compensated by maximal peripheral vasodilation
- the autoregulatory capacity is thus exhausted, and the blood vessels are unable to respond immediately with vasoconstriction when the perfusion pressure suddenly increases after successful CEA/CAS → tissue is therefore exposed to hyperemia
- hyperemia is usually defined as an increase in cerebral blood flow (CBF) >100% compared to the baseline → Cerebral blood flow regulation
- preoperative CBF value is usually unknown; the interhemispheric difference may be used (except in patients with contralateral stenosis)
- the risk of CHS can also be estimated using TCCD/TCD – a 1.5- or 2-fold postoperative increase in mean MCA flow velocity compared to baseline may predict the occurrence of CHS (Fujimoto, 2004)
- symptomatic CHS was also reported with a CBF increase of < 50%; in such cases, the term reperfusion syndrome should be preferred (, 2004)
- preoperative CBF value is usually unknown; the interhemispheric difference may be used (except in patients with contralateral stenosis)
- reperfusion injury (typically after acute stroke therapy) is usually caused by rapid normalization of flow within the infarcted tissue
- these patients have normal or only mildly increased CBF (20–44% above baseline)
- however, true hyperperfusion syndrome may also develop in acute stroke patients (Kneihsl, 2021)
- TCCD studies indicate that blood flow normalizes within one month after surgery; autoregulation is restored within approx. 6 weeks [Megee, 1992]
Hyperperfusion syndrome
clinical signs and symptoms + markedly increased CBF that exceeds the metabolic demand of the brain tissue, following the restoration of blood flow after a period of chronic hypoperfusion
Reperfusion syndrome
clinical signs and symptoms + normal or only mildly increased CBF occuring after acute stroke recanalization therapy
- reperfusion symptoms are increasingly recognized in various organs following revascularization procedures, such as:
- reperfusion arrhythmias
- gastrointestinal injury after reperfusion, leading to decreased intestinal barrier function
- marked polyuria following angioplasty in patients with renovascular disease
- reperfusion arrhythmias
| CHS risk factors |
|
Changes in the brain tissue resemble hypertonic encephalopathy:
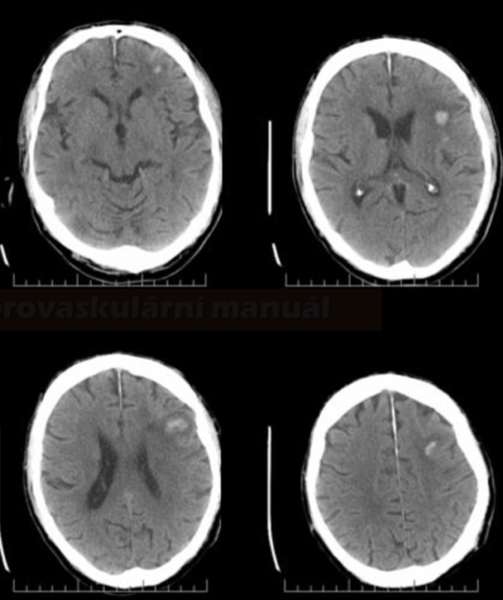
- cerebral edema
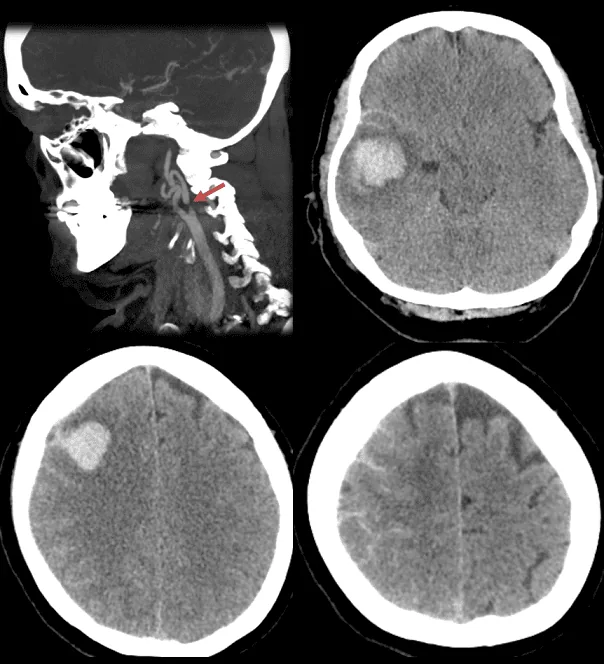
- intracerebral hemorrhage (ICH) /hemorrhagic transformation of ischemia
- intraventricular hemorrhage (IVH)
- subarachnoid hemnorrhage (SAH)
Clinical presentation
- severe headache
- worsening in the prone position
- ipsilateral to the lesion side or diffuse
- impaired consciousness
- epileptic seizures (often focal)
- focal neurologic deficit (usually with ICH)
Diagnostic evaluation
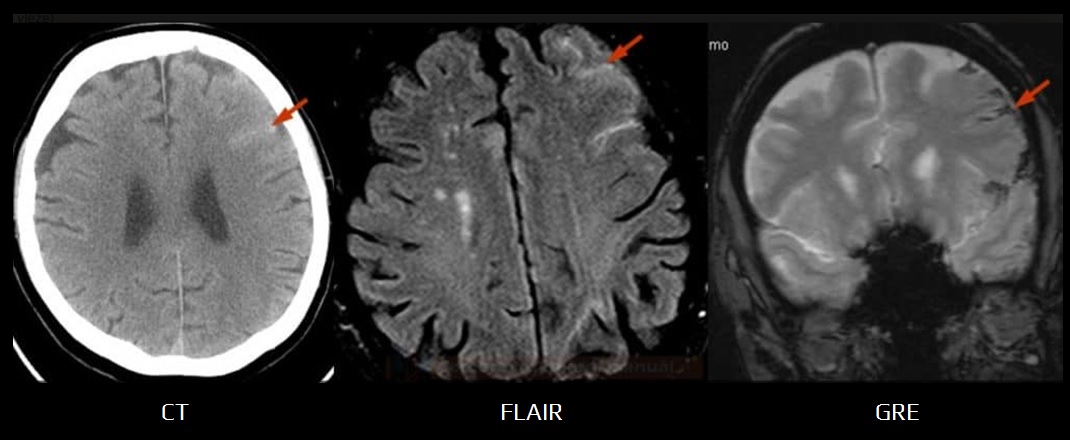
Parenchymal Imaging (CT/MRI)
- CT depicts edema/ICH/SAH in the territory of the recanalized artery
- edema is typically found in white matter (vasogenic), with or without mass effect
- bleeding can be petechial or even large parenchymal
- MRI findings may resemble those of PRES
- T2/FLAIR – diffuse hyperintense lesions
- T1C+ – possible leptomeningeal enhancement, not parenchymal
- hyperintense acute reperfusion marker (HARM) – delayed gadolinium enhancement of the cerebrospinal fluid space on FLAIR
(Cho, 2014)
- hyperintense acute reperfusion marker (HARM) – delayed gadolinium enhancement of the cerebrospinal fluid space on FLAIR
- DWI shows no lesions (typically, there is no cytotoxic edema)
- hemorrhage signal is age-dependent → MRI in hemorrhage diagnosis
Vascular imaging (CTA/neurosonology)
- CT angiography excludes thrombotic complications in the carotid artery and/or distal embolization
- TCD/TCCD shows an increased flow (↑PSV) and decreased resistance (↓PI and RI) in the MCA of >100% compared to the pre-intervention values
- an elevated PI suggests increased vascular resistance due to microembolization or intracranial hypertension
- an elevated PI suggests increased vascular resistance due to microembolization or intracranial hypertension
Perfusion imaging
- typical ipsilateral findings on CTP: ↑CBF, ↑CBV, ↓MTT
Prevention
- timing of the procedure
- benefit of CEA is greatest in the first 2 weeks following an ischemic event
- however, in cases of extensive ischemia, some delay is advisable
- perioperative TCD/TCCD monitoring
- enables early detection of hyperperfusion and the need for even stricter blood pressure (BP) control
- enables early detection of hyperperfusion and the need for even stricter blood pressure (BP) control
- strict blood pressure control
- BP reduction should be considered even in normotensive patients exhibiting hyperperfusion on TCD, as some may develop delayed hypertension
- the exact target value is unknown, and an individualized approach is advised (considering age, comorbidities, etc.)
- preferably, blood pressure should be lowered with drugs that do not increase CBF, such as labetalol and clonidine (avoid ACE-I, CCB, and especially vasodilators)
- free-radical scavengers – further trials are needed
Management
- rigorous blood pressure correction (ideally <120/80 mmHg) – it also serves as a preventive measure
-
in the case of seizure activity, administer antiseizure medication (ASM) – PHE, VPA, LEV → acute symptomatic seizures
-
start antiedema therapy
Management of patients with hemorrhagic transformation/SAH/ICH
- discontinue antiplatelet therapy and consider platelet concentrate infusion
-
administer (Solumedrol) 25-125 mg IV (to neutralize the effect of clopidogrel or other thienopyridines) [Qureshi, 2008]
- this approach is not standardized and is based on limited evidence
-
if the CT scan indicates no progression in 24 hours, patients with stents should recieve aspirin; clopidogrel therapy should be delayed for 5-7 days
Prognosis
- depends on timely recognition of hyperperfusion and adequate treatment of hypertension before cerebral edema or ICH develops
- the prognosis after ICH is poor (mortality of 36–63%, significant morbidity in the survivors)
- the prognosis of CHS in patients without ICH is much better, with low mortality
FAQs
- CHS is a potentially serious complication that can occur after procedures that restore normal blood flow to previously hypoperfused areas of the brain (CEA, CAS, etc)
- it is characterized by a significant increase in cerebral blood flow that can lead to brain edema, hemorrhage, and other neurological impairments
- CHS often occurs after procedures like carotid endarterectomy or carotid artery stenting, especially in patients with a significantly reduced cerebral blood flow and poor cerebral vascular reserve detected before the procedure (via CTP, TCD/TCCD)
- key risk factors include hypertension, intraoperative or postoperative fluctuations in blood pressure, severe carotid artery stenosis, recent TIA or stroke, and older age
- common symptoms include severe headache, seizures, focal neurological deficits (such as hemiparesis), and altered consciousness
- these symptoms typically manifest within the first week post-procedure but can occur later
- the diagnosis is primarly clinical, supported by imaging findings (MRI/CT showg cerebral edema without evidence of cerebral infarction
- TCD/TCCD can be used to monitor arterial flow velocities, which may indicate hyperperfusion
- treatment involves managing symptoms, strict blood pressure control, and sometimes the use of corticosteroids to reduce cerebral edema
- in cases of severe brain edema or hemorrhage, neurosurgical intervention may be required
- careful patient selection, preoperative assessment of cerebral vascular reserve, intra- and postoperative monitoring of cerebral blood flow, and meticulous postoperative blood pressure management
- the duration of CHS can range from days to weeks in its acute phase, with potential for longer-term effects in severe cases or when complications arise
- the prognosis varies depending on the severity of initial symptoms and the timeliness of treatment; with prompt recognition and management, many patients can recover fully
- however, severe cases involving extensive brain edema or intracerebral hemorrhage can lead to significant morbidity or mortality (such as cognitive impairment, motor function abnormalities, or epilepsy)