ISCHEMIC STROKE / CLASSIFICATION AND ETIOLOGY
Arterial dissection
Updated on 07/06/2024, published on 05/05/2023
Definition and pathophysiology
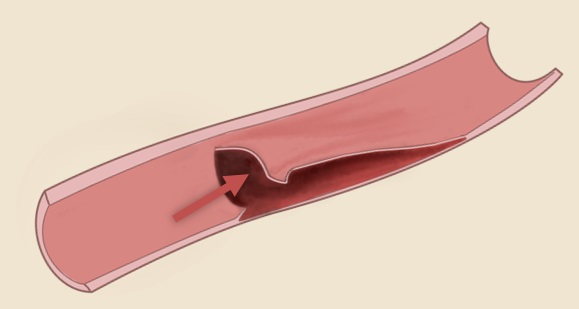
- arterial dissection is characterized by a tear in the inner layer of the arterial wall
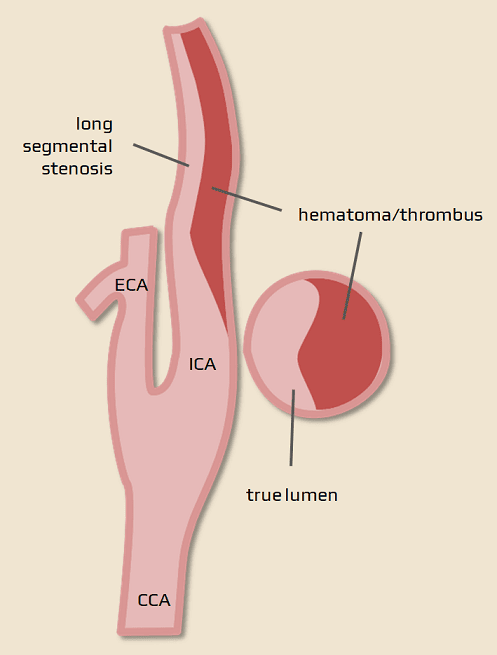
- blood enters the tear and separates the wall layers – a false lumen is formed alongside the true lumen
- the false lumen often becomes thrombosed, leading to stenosis or occlusion of the affected artery (diagnosing dissection can be challenging when occlusion is present)
- thrombus growth and/or arterio-arterial embolization are frequently observed if extracranial arteries are involved
- dissecting aneurysm (pseudoaneurysm) may also develop

- rupture through the adventitia leads to bleeding (causing SAH in intracranial lesion)
Epidemiology
- dissection mainly affects younger and middle-aged individuals
- accounts for ∼ 5% of strokes in the age group < 45 years
- rare cause of stroke in the elderly population
- incidence is increasing due to better imaging techniques
- carotid arteries are most commonly involved (∼ 80% of cases; typically, the lesion is located below the skull base), while vertebral arteries are involved in ∼ 20% of cases (primarily at the level C1-2 – atlas loop)
- more than one vessel is affected in ∼ 25% of cases
- more than one vessel is affected in ∼ 25% of cases
- intracranial dissection is relatively rare
- dissection poses the highest risk of stroke within the first 14 days (absolute risk increase is ~1.25%, decreasing to 0.18% in weeks 3-4) [Morris, 2017]
Etiology
- spontaneous x traumatic (trauma or C-spine manipulation)
- in both cases, the arterial wall or some of its layers is presumed to be defective
- in both cases, the arterial wall or some of its layers is presumed to be defective
- abrupt hyperextension/rotation of the head causes tension of the carotid artery over the transverse processes of C2, C3, or the lateral process of the atlas, resulting in tearing of the intima. Conversely, the carotid artery is wedged between the mandible and C1 after extensive and rapid flexion
- similarly, the vertebral artery is usually bruised over the edges of the transverse foramina or the atlas arch
- if spontaneous dissection is suspected, renal artery imaging (in addition to cerebral artery imaging) is recommended to exclude Fibromuscular dysplasia
- rare conditions include Eagle´s syndrome
- associated with the elongation of the styloid process or calcification of the stylohyoid ligament, clinically characterized by throat and neck pain radiating into the ear
- rarely, it may cause carotid dissection (Ogura, 2015) (Saccomanno, 2018)
| Some conditions associated with arterial dissection | |
|
Fibromuscular dysplasia (FMD)
Syphilitic angiopathy Proliferative inflammation in some forms of arteritis Moya-moya Connective tissue disorders (Marfan syndrome, Ehlers-Danlos syndrome) |
Clinical presentation
Extracranial dissection
Carotid dissection
- unilateral neck, head, or orbital pain
- Horner’s syndrome (caused by damage to the adventitial sympathetic plexus)
- Harlequin sign (hyperhidrosis and flush of the contralateral half of the face during physical exertion)
[Drexler, 2014]
- lesion of cranial nerves IX-XII (compressed by the dissecting aneurysm) [Kasravi, 2010]
- may imitatate brainstem lesion
- stroke symptoms in ~ 2/3 of cases
- pulsatile tinnitus (DDx of CCF or dural AV fistula)
- symptoms of carotid dissection can range from asymptomatic carotid stenosis/occlusion to extensive territorial stroke
- ipsilateral neck pain
- a combination of extra- and intracranial involvement is common
- symptoms depend on the extent of dissection, particularly whether the basilar artery or spinal arteries are involved, and the status of the collaterals
- dissection without concomitant distal embolization might remain asymptomatic if the contralateral vertebral artery is fully functional
- spinal cord ischemia results from the occlusion or hypoperfusion of spinal arteries
Aortic arch dissection → see separate chapter
- dissection may be a source of emboli or lead to stenosis/occlusion of the aortic branches (most commonly CCA and ICA)
- presents with:
- sudden onset of severe chest pain that radiates between the shoulder blades (often initially mistaken for a coronary event).
- hypotension, sometimes with transient loss of consciousness
- asymmetrical or very weak pulses in the neck and upper limbs
- cardiac tamponade may occur, necessitating urgent surgery
Intracranial dissection
- intracranial dissections are rare in the absence of systemic connective tissue disease
- predominantly occurring in the vertebrobasilar territory and the supraclinoid portion of the ICA
- headache typically occurs at the onset, almost always followed by the development of a neurological deficit
- in extracranial dissections, the development of a neurological deficit is gradual, often occurring several days after the initial headache; asymptomatic lesions are not uncommon
- the main mechanism is hemodynamic failure behind the occlusion or stenosis (including occlusion of perforators); hemodynamically induced fluctuation of the neurological deficit is frequent
- while distal embolization predominates in extracranial dissections
- while distal embolization predominates in extracranial dissections
- intracranial dissection may also lead to subarachnoid hemorrhage
- diagnosis is challenging; angiographic findings are often nonspecific (occlusion or stenosis); typical features (string sign, double lumen) are usually not visible
- intracranial dissection may be confused with vasculitis
- involvement of the ICA may be suggestive of giant cell arteritis, which predominantly affects the petrous and cavernous segments
- intracranial dissection usually has a worse prognosis than extracranial dissection (due to limited potential for collateral circulation)
Diagnostic evaluation
- a typical history of head/neck pain after C-spine injury or manipulation, followed by the development of a neurologic deficit
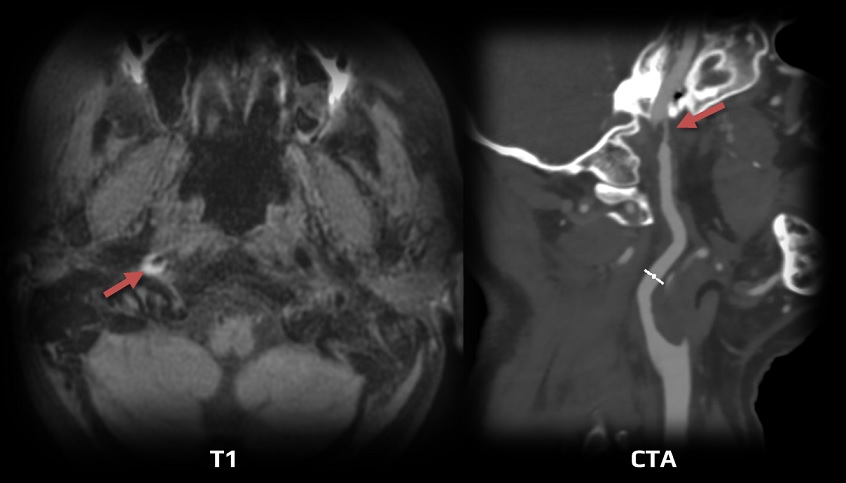
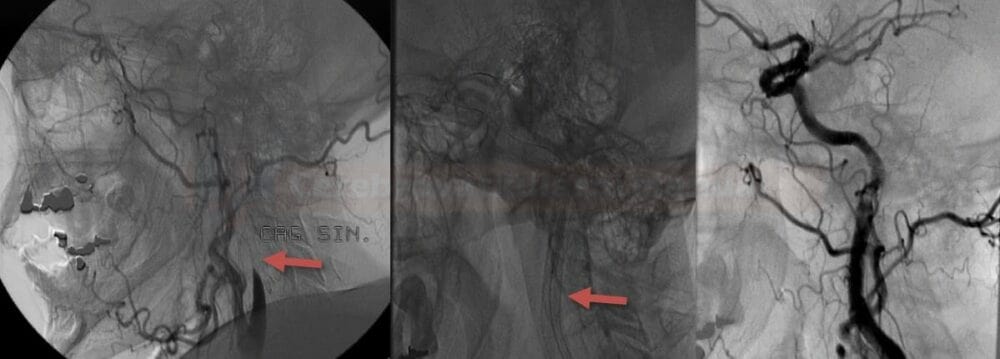
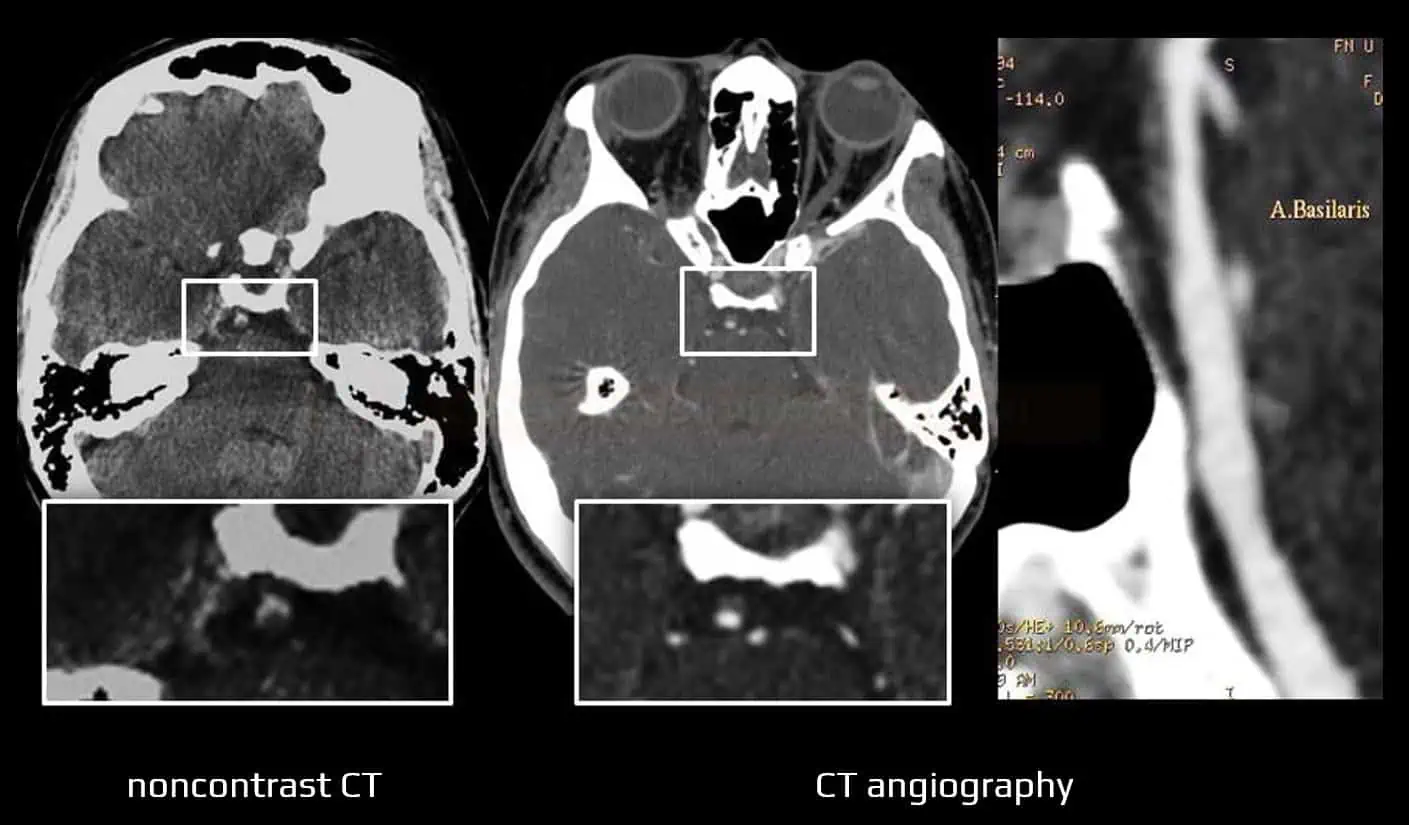
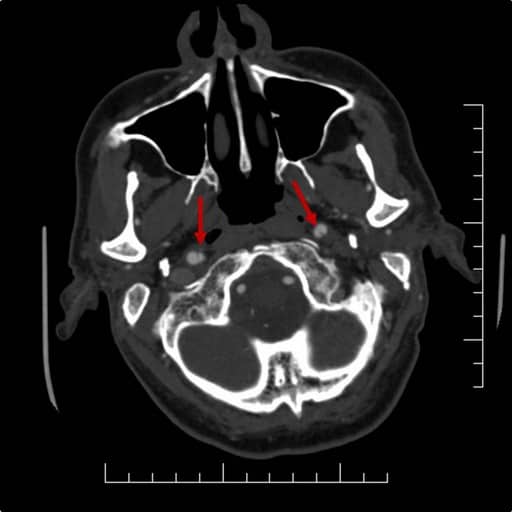
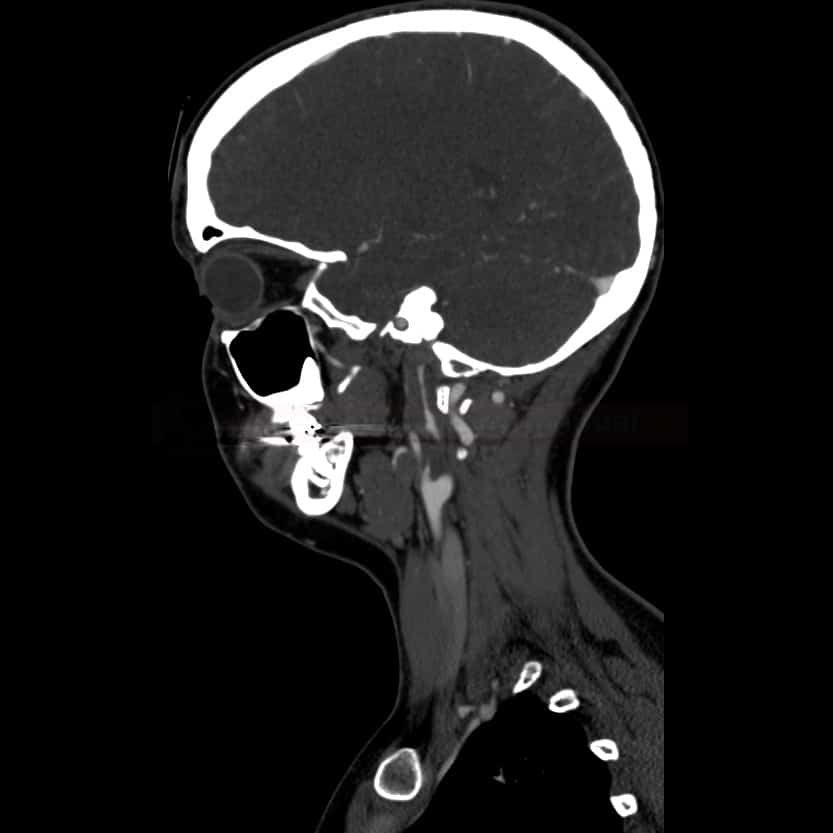
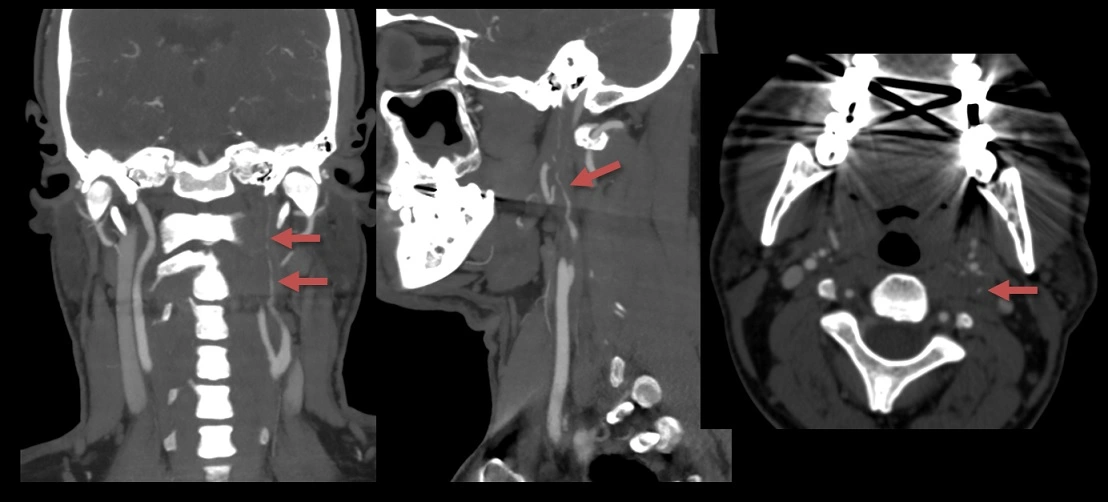
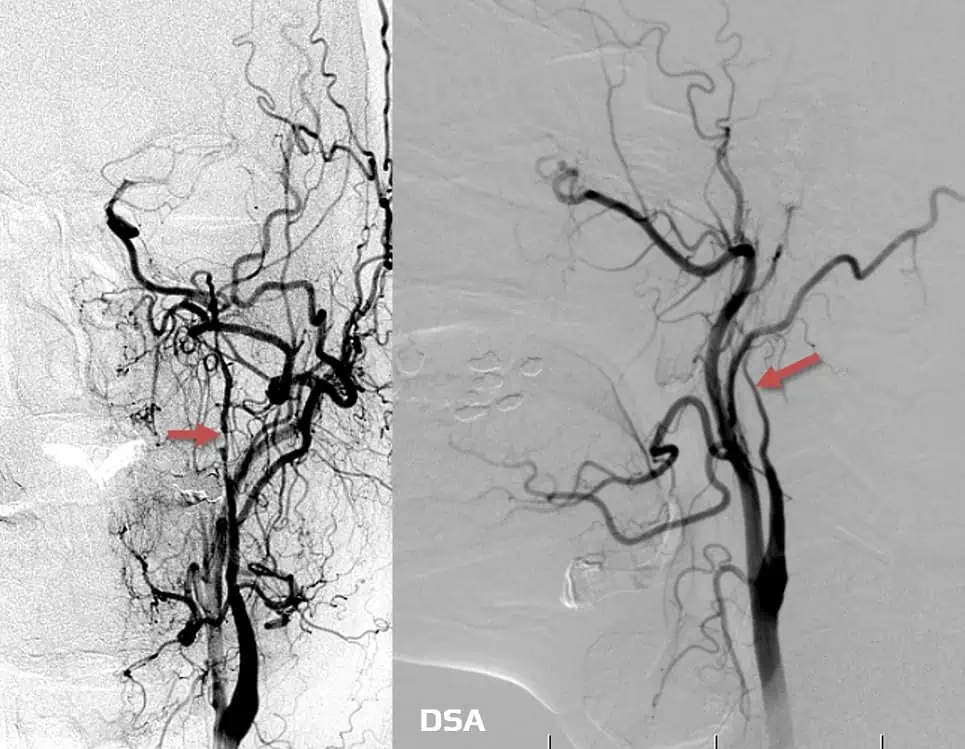
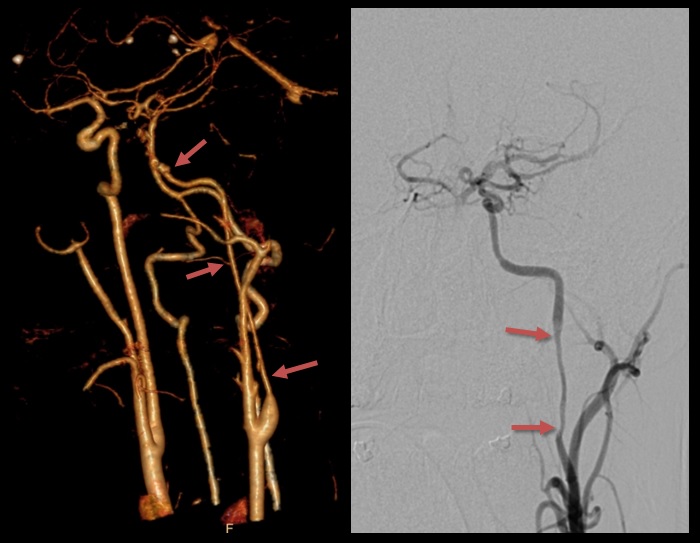
- vascular imaging (neurosonology, CTA/MRA/DSA) is essential, and these findings are typical:
- a smooth, convex, elongated enlargement of the vessel wall causing luminal narrowing (string sign); stenosis may terminate relatively abruptly at the distal end
- a double lumen (a pathognomonic finding)
- a dissecting aneurysm
- diagnosis of intracranial dissection is problematic because:
- finding of nonspecific segmental stenosis or occlusion is common – may be confused with partially recanalized thrombus, thrombotic occlusion, or vasculitis
- typical string sign is often absent
- MRI detection of evident intramural hematoma on the transverse section is less probable
- artery fenestration must be differentiated
Neurosonology
| Content available only for logged-in subscribers (registration will be available soon) |
CT angiography
Digital subtraction angiography
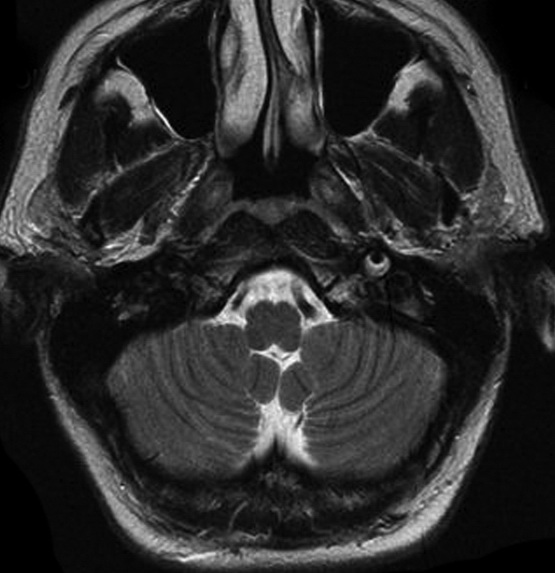
Magnetic resonance imaging (MRI)
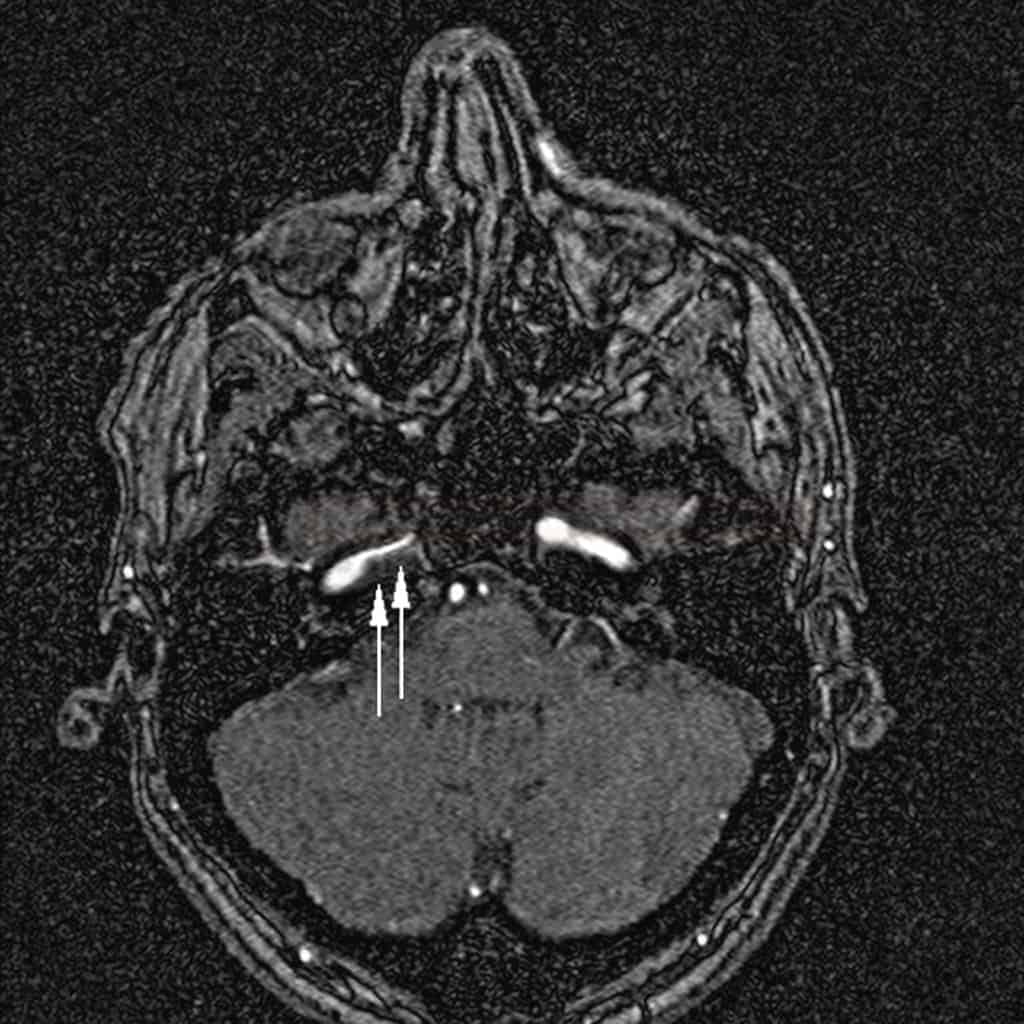
- the image changes over time (through acute, subacute, and chronic phases) – it is necessary to evaluate fat- and blood-suppressed T1, T2, and TOF simultaneously [Habs, 2011]
- in the subacute phase, the T1 sequence shows a thrombus in the false lumen (T1 hyperintense ring)
- MRA shows a luminal narrowing and possibly a thrombus in the false lumen
Management
- treatment strategy for dissection-related stroke should be individualized
- treatment options include:
- thrombolysis
- endovascular treatment
- anticoagulant/antiplatelet therapy (SAPT or DAPT)
Thrombolysis in dissection-related acute stroke
- dissection was not listed as a contraindication in the NINDS or ECASS II trials, but the number of enrolled patients is unknown
- according to published series of patients treated with IVT and IAT, efficacy and safety appear to be similar to those with other stroke etiologies [Biller, 2014] [Nedeltchev, 2002] [Georgiadis, 2006]
- it remains uncertain whether thrombolysis (as well as anticoagulation) might contribute to the progression of intramural hematoma
- IVT may be considered for isolated cervical dissection (ESO guidelines 2021)
- IVT should preferably not be used for intracranial dissection (ESO 2021 – expert consensus)
- IVT is absolutely contraindicated for aortic dissection
Endovascular therapy
- stenting may be indicated in patients who experience a recurrent stroke despite the best medical therapy (BMT) (AHA/ASA 2021 2b/C-LD) [Biller, 2014]
- in the acute phase, the endovascular procedure may be performed in:
- patients with a tandem lesion (ICA dissection + intracranial occlusion – the procedure appears to be safe [Marnat, 2020]
- patients with extracranial ICA dissection, particularly if a tight stenosis or occlusion causes symptomatic hypoperfusion (demonstrated by TCD/TCCD or CTP)
- patients with intracranial dissection resulting in hypoperfusion (e.g., due to a progressive stenosis)
- dual antiplatelet therapy (ASA+CLP) is required before stenting and should be continued for 3 months
- self-expanding stents are preferred
- multiple stents may be necessary; start distally and ensure that stents partially overlap
- thromboembolic complications can be treated mechanically with IAT or IIb/IIIa inhibitors
- progressive pseudoaneurysms can be managed with a stent or stent graft
Antithrombotics
- anticoagulant or antiplatelet therapy should be continued for at least 3 months (AHA/ASA 2021 1/C-EO)
- although the primary mechanism involves arterial wall bleeding, the predominant mechanism of stroke is distal thrombotic embolization
- studies comparing the effects of antiplatelet and anticoagulant therapy have shown no difference in efficacy in preventing recurrent stroke
- CADISS (Cervical Artery Dissection In Stroke) remains the only randomized trial [Hugh, 2019]
- n=250, 124 (anticoagulation) vs 126 (antiplatelet), stroke + death/3 months: 1% vs 2% (p=0.63)
- non-randomized trial following 298 patients with carotid dissection showed similar results for aspirin and anticoagulants [Georgiadis, 2009]
- TREAT-CAD study is ongoing; it should demonstrate the noninferiority of ASA versus warfarin
- CADISS (Cervical Artery Dissection In Stroke) remains the only randomized trial [Hugh, 2019]
- intracranial dissections carry a higher risk of SAH due to the specific wall configuration (narrow media, absence of lamina externa), favoring antiplatelet therapy
- anticoagulation is not appropriate in patients with larger infarcts (↑ bleeding risk)
- comparison between monotherapy (SAPT) and dual antiplatelet therapy (DAPT) is not available
Anticoagulants
- administer for 3 months, then switch to ASA
- some authors recommend repeating the vascular imaging in 2-3 months and adjusting the duration of anticoagulation based on the results
- complete recanalization allows an earlier switch to ASA
- persistent stenosis and luminal irregularities may warrant continued anticoagulation for up to 6 months
Antiplatelets
- aspirin 75-100mg daily is the most commonly used antiplatelet
- short-term DAPT (ASA+CLP) may be considered
- antiplatelet therapy should be continued for at least 3 months
- it is unclear whether long-term antithrombotic therapy should be continued in patients without connective tissue disease.
- probably not; studies are not available
- likely/possible indications for prolonged antiplatelet therapy: [Biller, 2014] :
- residual stenosis or persistent occlusion (evidence of benefit is lacking)
- patients with connective tissue disease and recurrent dissections
- pseudoaneurysm sac thrombosis
Other measures
- standard treatment of vascular risk factors (especially maintaining normal blood pressure)
- oral contraception is not recommended
- avoid statins unless indicated for another medical reason
- avoid neck manipulation (e.g., massage, chiropractic manipulation), extreme head and neck movements or posture, heavy lifting, and sport activities such as contact sports and skiing
- if Eagle´s syndrome is detected, surgical removal of the elongated styloid process should be considered (Selvadurai, 2022)
Follow-up and prognosis
- prognosis varies based on the location and extent of the initial injury, timely intervention, and response to treatment
- one-third of patients with cervical artery dissection who presented with occlusion experience resolution at follow-up, with a median time to healing of 4 months
- healing can continue up to 12 months after the initial dissection
- there is no correlation between chronic residual arterial disease and stroke rate beyond the first 6 months
- pseudoaneurysm can resolve or decrease in size, some may increase in size or develop de novo later on (risk is higher with multiple dissections)
- dissecting aneurysms usually have a benign prognosis and are not associated with an increased risk of recurrent stroke or rupture
- aneurysms can enlarge and rarely cause compressive symptoms, such as dysphagia, hoarseness, and stridor
- early diagnosis and prompt intervention generally lead to better outcomes
- regular follow-up is essential for monitoring and addressing potential complications

![Different stages of an ICA dissection on MRI [Habs, 2011] Different stages of an ICA dissection on MRI [Habs, 2011]](https://www.stroke-manual.com/wp-content/uploads/2022/05/dissection-on-MRI-stages.jpg)