ISCHEMIC STROKE / PREVENTION
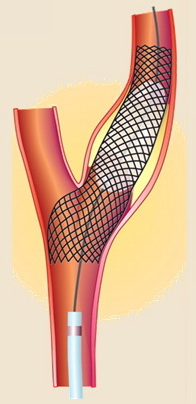
Carotid angioplasty with stenting (CAS)
Updated on 27/03/2024, published on 27/01/2022
- ~ 10-20% of ischemic strokes are caused by significant carotid stenosis (TOAST 1)
- the risk of stroke or vascular death with symptomatic ICA stenosis >70% is up to 26%/2 years, depending on symptoms, gender, and severity of stenosis [Rothwell, 2005]
- risk of stroke in asymptomatic carotid stenosis of >60% is ~11%/5 years
- increased risk is associated with high-risk plaque characteristics and a higher degree of stenosis
- revascularization procedures reduce the risk of stroke but are beneficial only when the perioperative risk is < 6% (symptomatic stenosis) or 3% (asymptomatic stenosis) → indications for revascularization are discussed here
- endovascular treatment (carotid angioplasty and stenting – CAS) is an alternative to carotid endarterectomy (CEA)
- since 1994, there have been significant improvements in technology (including proximal and distal embolic protection devices – EPD) and experience with carotid stenting
- new coated stents promise additional safety → see here
- Roadsaver, CGuard, Gore stent
- randomized trials showed similar efficacy/safety of stenting and endarterectomy (e.g., SAPPHIRE and CREST)
- trials were designed to demonstrate non-inferiority and focused on patients with high surgical risk due to comorbidities (CAD, heart failure, pulmonary disease, etc.)
- CAS is not more risky than CEA and prevents at least the same number of strokes as CEA
- patients with extensive white matter lesions on CT/MRI (ARWMC score ≥ 7) should undergo CEA (ICSS trial)
| Trial | number of patients |
30-days mortality/morbidity |
long-term mortality/morbidity |
| CARESS (2003) | 397 | 2 vs. 3% | – |
| CAVATAS (2001) | 504 | 10 vs 10% | 14.3 vs. 14.2 |
| SAPPHIRE (2004) | 334 | 4.4 vs. 9.9% (stroke+MI+death) |
12.2 vs. 20.1 |
| CREST (2010) | 2502 | 5.2 vs. 4.6% (CEA) (stroke+MI+death) 4.1% vs 2.3% (CEA) (perioperative stroke) |
primary endpoint: 7.2 vs 6.8% / 4 years stroke+death 6.4 (CAS) vs 4.7% (CEA) |
Indications for stenting
The primary indication for CAS (when CEA cannot be performed)
|
Contraindications
- unfavorable access-vessel anatomy
- significant tortuosity and/or severe atherosclerosis of the aortic arch or proximal CCA/BCT that prevent safe catheter insertion
- bovine arch – the most common variant of the aortic arch – the brachiocephalic (innominate) artery shares a common origin with the left CCA
- tortuous iliac artery
- unfavorable cerebral vessel features
- large calcified plaques preventing the arterial remodeling with stent
- distal ICA diameter < 3 mm preventing the use of distal protection (relative contraindication, proximal protection can be used)
- intracranial aneurysm > 5 mm or AV malformation (relative contraindication)
Procedure
- before performing the procedure, inform the patient about the risks, benefits, and alternatives of the procedure; the patient must sign an informed consent form
- order standard laboratory tests (CBC, coagulation test, basic metabolic panel) and other tests required by an anesthesiologist
- insert IV cannula and short-term urinary catheter and shave the groin (if transfemoral access is used)
Premedication
- proper medical prevention of thrombotic complications is critical
- start ≥ 3 days before the elective procedure
- premedication is not standardized; different regimens have been used in clinical trials
- aspirin 300mg + clopidogrel 75 mg (SAPPHIRE trial) for 3 days
- aspirin 2x300mg + clopidogrel 2×75 mg (CREST trial) for 3 days
- aspirin 100mg + clopidogrel 75 mg for 3 days
- if clopidogrel is contraindicated, ticlopidine 2×250 mg or ticagrelor can be used (there are no hard data for this recommendation)
- in the acute setting,600mg of aspirin and 4-6 clopidogrel tablets (300-450mg) should be administered at least 4 h before the procedure (antiplatelet therapy in acute stroke patients treated with IVT + CAS is discussed here)
- follow guidelines for the prevention of contrast-induced nephropathy
Periprocedural monitoring
- the procedure is performed in cooperation with an experienced neuroradiologist, anesthesiologist, and a neurologist
- analgosedation is preferred
- basic monitoring:
- ECG and blood pressure (every 5 minutes)
- correct BP correction is critical throughout the procedure (prevention of hyperperfusion syndrome) → parenteral antihypertensives
- if bradycardia occurs, it may be necessary to administer ATROPIN 0.5 mg IV (which may also be given prophylactically)
- a pulse oximetry
- patient’s neurological status (level of consciousness, speech, visual field, acral motor skills)
- ECG and blood pressure (every 5 minutes)
- TCD/TCCD monitoring (optional)
- hemodynamic information (hyperperfusion syndrome is associated with a significant increase of blood flow velocity in the MCA during the procedure, with a concomitant decrease of PI and RI)
- detection of embolization
- microembolization (MES)
- during contrast administration (dissolved air bubbles)
- detection of particles released during critical phases of the procedure (distal protection filter insertion, pre-dilatation, stent deployment, post-dilatation, and protection removal)
- increased risk of stroke with > 5 series of microembolizations
- macroembolization leads to transient or permanent flow restriction in the occluded vessel
- microembolization (MES)
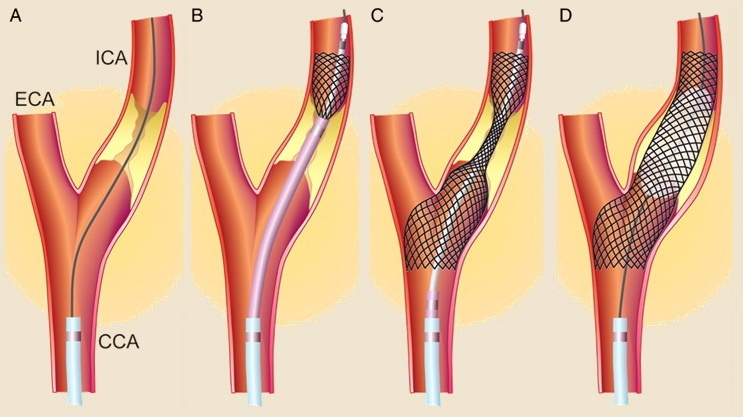
CAS technique
- angioplasty – an inflatable balloon is used to dilate the narrowed area
- angioplasty with secondary stenting – angioplasty is performed with an inflatable balloon, followed by stent placement
- primary stenting with a balloon-expandable stent – after insertion, the stent is expanded by balloon dilatation
- primary stenting with a self-expanding stent (preferred)
- less traumatic and safer than angioplasty alone due to a reduced risk of distal embolization, plaque rupture, or arterial dissection
- predilate lesion only if stenosis does not allow stent insertion; use a balloon smaller than the lumen diameter to achieve a lumen > 2.5 mm
- after stent deployment, post-dilatation is performed if a satisfactory result is not achieved or if the stent does not fully adhere to the arterial wall (contrast is visible between the wall and the stent)
- post-dilatation may lead to higher morbidity/mortality ⇒ perform in severe residual stenosis only [Obeid, 2015]
- self-expanding stents are less prone to deformation
- less traumatic and safer than angioplasty alone due to a reduced risk of distal embolization, plaque rupture, or arterial dissection
- it is recommended to use embolic protection devices (EPDs)
- distal (both F-EPD and DO-EPD seem to be equally effective)
- filter EPD (F-EPD)
- distal occlusive EPD (DO-EPD)
- proximal
- no need for a suitable landing zone
- may be safer for highly embolic lesions
- distal (both F-EPD and DO-EPD seem to be equally effective)
- medication during procedure
- heparin with ACT > 250s
- aggressive hydration
- vasopressors if significant hypotension occurs
- atropin IV
- if bradycardia occurs during dilatation
- prophylactic administration (problematic in patients with significant CAD)
Post-procedural management
- retrieve filter (aspirate filer prior to retrieval if slow flow occurs)
- remove the sheath and secure the groin (use Angioseal or other devices)
- observe the patient in the ICU
- continue monitoring vital signs with careful BP control (check every 15 minutes for the first hour, then every 30 minutes for at least 24 hours)
- careful correction of BP is the key to preventing hyperperfusion syndrome
- careful correction of BP is the key to preventing hyperperfusion syndrome
- check for complications and monitor neurological status (GCS, NIHSS)
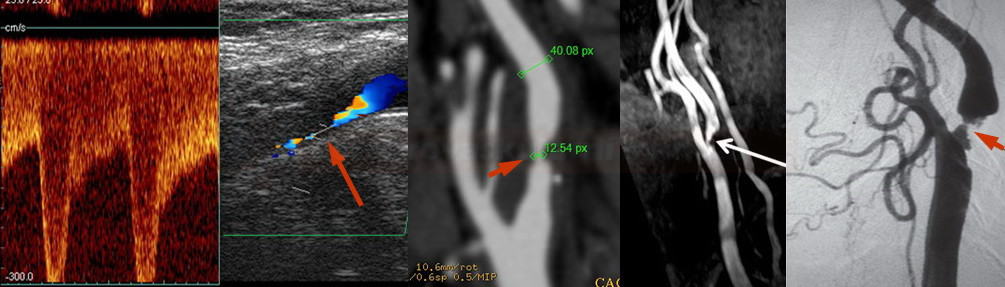
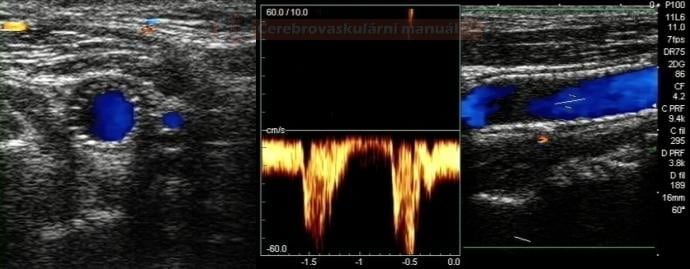
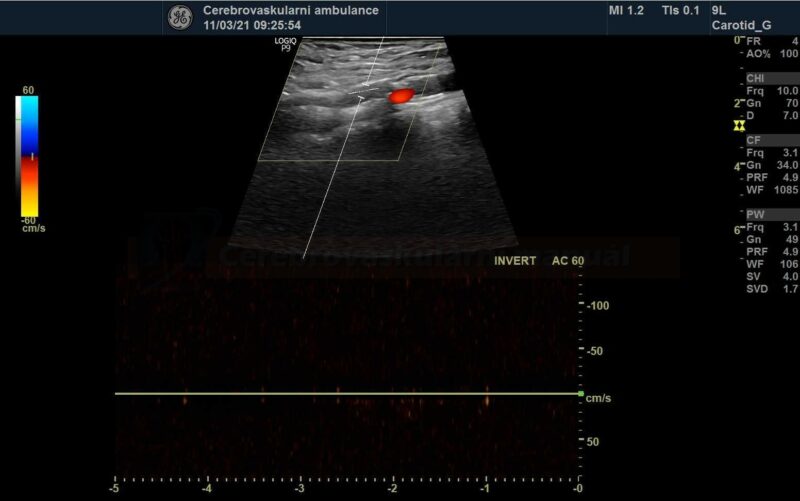
- perform control Doppler ultrasound within 24 hours
- record PSV in the proximal, mid, and distal portions of the stent and in the segment distal to the stent
- stent artifactually increases flow velocities
- after the procedure, continue dual antiplatelet therapy (DAPT; aspirin 100 mg + clopidogrel 75 mg) for at least 4 weeks (1-3 months)
- then switch the patient to monotherapy (either ASA or CLP)
Complications
- only complications related to stenting are discussed below
- common adverse events related to endovascular procedures and diagnostic DSA are discussed elsewhere → overview of complications during endovascular procedures
|
Central complications
|
|
 |
1.1 Distal complications
|
|
|
| 1.2 In-stent complications |
|
| 1.3 Proximal complications | |
|
|
| Peripheral (access-site) complications Femoral arterial access is commonly used for CAS, but radial or brachial approaches may be considered. Complications range from asymptomatic to life-threatening conditions |
|
 |
|
| Systemic complications | |
|
|
- microembolization (most common) x microembolization
- responsible for a substantial portion of the 30-day mortality/morbidity
- distal and proximal protection devices prevent cerebral embolization
- a meta-analysis of approximately 2400 patients showed that the 30-day mortality and morbidity were 5.5% and 1.8% (without and with protection) [Kastrup, 2003]
- use of distal protection is debated; many centers have comparable results without it
- its insertion is associated with additional manipulation in carotid bifurcation, which itself increases the risk of embolization or vasospasm
- other complications, such as filter thrombosis, stent entrapment, and damage, must be considered
- mechanical recanalization or intraarterial thrombolysis may be performed in the case of symptomatic distal embolization
- the main mechanism of acute stent thrombosis is the platelet activation
- the highest risk is in the first 72h
- the incidence of acute and subacute carotid stent thrombosis is 0.5-2%
- in addition to occlusion, mural thrombi can occur and embolize to the brain
- any change in the patient’s neurological status after the procedure should raise suspicion of acute stent thrombosis and requires urgent diagnosis (most easily by duplex ultrasound) and therapy with Ilb/IIIa receptor inhibitors
- proper premedication (see above) and stent placement are key to the prevention of such complication
- prophylactic add-on therapy with IV abciximab reduces the risk of ischemic complications while increasing the risk of bleeding
- prophylactic add-on therapy with IV abciximab reduces the risk of ischemic complications while increasing the risk of bleeding
- therapy → IV or IA abciximab
(Integrilin) usually 1mL/2mg or 1mL/0.75 mg
- IV bolus: 0.2 mg/kg within 5 minutes, followed by infusion (Sedat, 2014)
- IV infusion: 5mL(10mg) + 45mL of NS (1mL=0.2 mg) …… 3mL/h (0.6 mg/h) 0.125ug/kg/min (max 10ug/min!)
- IA bolus: 5mL /10mg) + 45 mL of NS (1mL=0.2 mg) …… bolus 10 mL (2mg) every 5 minutes till max dose 10 mg
(Reopro) 1mL/2mg
- a monoclonal anti-glycoprotein IIb/IIIa receptor antibody
- IV bolus: 0.25 mg/kg within 5 minutes, followed by infusion
- IV infusion: 5mL (10mg) + 45mL NS (1mL of solution = 0.2 mg) …… 3mL/h (0.6 mg/h) 0.125ug/kg/min (max rate 10ug/min!)
- IA bolus: 5mL (10mg) + 45 mL NS (1mL of solution = 0.2 mg) …… bolus 10 mL (2mg) every 5 minutes, max dose 10mg
- endovascular microsnare loops have been developed to remove migrated stents (stentectomy) (Oh, 2012)
- deformation is more common with balloon-expandable stents compared to self-expanding stents
- uncorrectable deformation may require surgical revision
- stent fracture may result in dissection with possible formation of a pseudoaneurysm
- difficulties with distal protection retrieval can usually be resolved percutaneously
- careful manipulation is crucial
- it may be helpful to move the patient’s head, have the patient swallow, or choose a more flexible retrieval sheath. In case of filter fracture, it is recommended to ‘jail’ the filter against the arterial wall with an additional stent
Restenosis
Follow-up
- neurosonology – method of choice; always check the velocities within the stent as well as in the proximal and distal segments
- MR angiography may be subject to artifacts
- CT angiography