ISCHEMIC STROKE / STROKE PREVENTION
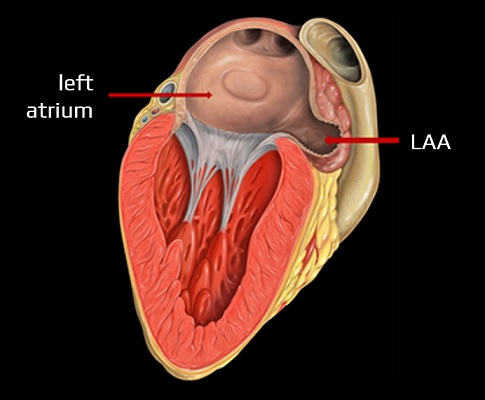
Left Atrial Appendage Occlusion (LAAO)
Updated on 29/02/2024, published on 14/12/2021
- atrial fibrillation (AFib) is a leading preventable cause of ischemic stroke
- the risk of stroke in individuals with AFib can be stratified using prediction tools, such as CHA2 DS2–VASc
- early detection of AFib (especially its paroxysmal form) and treatment with anticoagulants are critical
- oral anticoagulant therapy (OAC) carries an increased risk of bleeding and may be contraindicated for certain patients
- the few absolute contraindications to oral anticoagulation include active serious bleeding, associated comorbidities (e.g. severe thrombocytopenia <50 platelets/lL, severe anemia under investigation, etc.), or a recent high-risk bleeding event such as intracranial haemorrhage
- most patients who would be historically considered unsuitable for OAC therapy with VKA now seem to do relatively well on DOACs and unncessary withholding OAC is likely to result in undertreating the risk of stroke related to atrial cardiomyopathy
- endovascular or surgical treatment of the left atrial appendage (LAA) has been suggested as an alternative to anticoagulant therapy for true high-risk patients
- the rationale for this procedure is that a significant proportion of AFib thrombi form in the LAA (57% of valvular AFib and 91% of NVAFib).
- the procedure is also recommended by the guidelines
- stroke/TIA patients with NVAF who have contraindications to lifelong anticoagulation, but can tolerate it for at least 45 days, it may be reasonable to consider percutaneous closure of the LAA to reduce the risk of recurrent stroke and bleeding (AHA/ASA guidelines 2021 2b/B-R)
- ESC guidelines 2020 IIb/B
- clinical trials: PROTECT AF, PREVAIL
- real-world data: registry EWOLUTION and LAAO
| Content available only for logged-in subscribers (registration will be available soon) |
LAA occlusion methods
Endovascular LAAO
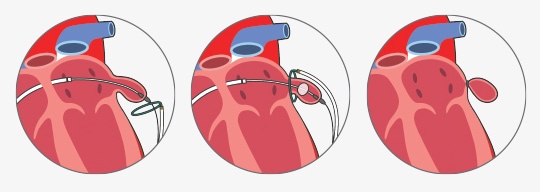
- all percutaneous LAA occluder implantation procedures require peripheral venous access (typically via the right femoral vein)
- a transseptal puncture is then performed to gain access to the left atrium and occluder device is deployed (Watchmen, Amplatzer, Amulet)
- in the Lariat procedure, a magnet link is created between an endocardial wire and pericardial wire, followed by epicardial suture ligation of the LAA
- regardless of the type of the closure procedure, transesophageal echocardiography (TEE) is essential for preprocedural screening, intraprocedural guidance, and postprocedural follow-up
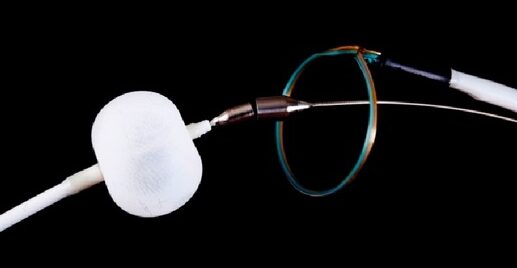
Watchman → see here
- made of a self-expanding nitinol frame and a permeable polyester cover
- Watchman device has the most available outcomes data
- FDA-approved (2015)
Amplatzer Amulet (Abbot) → see here
- LAA Occluder consists of a lobe and disc connected by a flexible waist and is constructed of a nitinol mesh covered with a polyester patch
- according to registries, the risk of periprocedural complications is up to 4.8%, and the subsequent risk of occluder thrombosis is 1.5-5% [Mandrola, 2017]
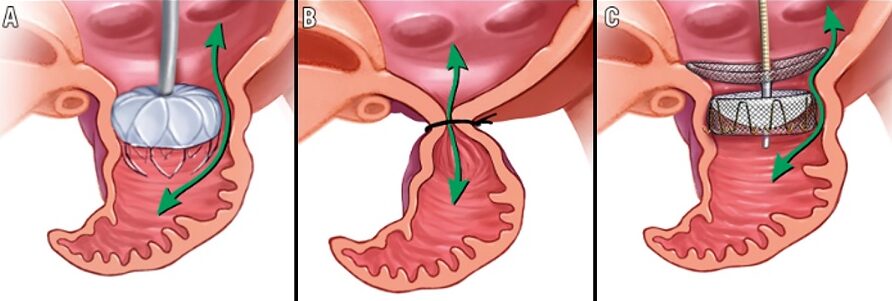
Lariat system → see here
- FDA-approved, capable of eliminating even larger LAAs
- closure with the Lariat device consists of both endocardially and epicardially delivered magnetic tipped wires that unite at the distal LAA wall
- once adequate LAA ligation is confirmed by echocardiography, the endocardial balloon catheter and wire are removed
- finally, the epicardial suture is released from the snare and tightened, which excludes the LAA
- a high risk of complications has been reported (14.7%)! [Price, 2014]
Surgical LAAO
- surgical LAA occlusion may be performed concurrently during cardiac surgery (ESC guidelines 2020 IIb/C)
- benefit has not been reliably assessed, but positive results with reduced mortality and stroke risk have been reported (Yao, 2018)
Periprocedural antithrombotic therapy
| Content available only for logged-in subscribers (registration will be available soon) |
Complications
- some authors report complications in up to 20% of cases [Fauchier,2018]
- the incidence of DRT in patients with LAA imaging was 7.2% per year
- according to the LAAO registry, major periprocedural complications occur in 2.16% of patients [Freeman, 2020]
- most commonly, pericardial effusion requiring intervention was reported (1.39%)
- stroke (0.17%) and death (0.19%) were rare
- follow-up TEE may detect incomplete LAA closure (leak)
- the annual risk of DRT is:
- 4-8% [Fauchier,2018]
- 4.1%, according to the EWOLUTION registry [Boersma, 2019]
- DRT is strongly associated with a higher risk of ischemic stroke during follow-up
- therefore, temporary antithrombotic treatment is required after the procedure (until the occluder is covered with endothelium)
- there is no universal antithrombotic protocol; the regimen is mostly derived from trials (see above)
- there is no universal antithrombotic protocol; the regimen is mostly derived from trials (see above)
- main risk factors for DRT according to the LAAO-DRT registry:
- permanent AFib
- pericardial effusion (pathological accumulation of fluid in the pericardial sac)
- hypercoagulable state
- renal insufficiency (CKD)
- deep implant position (>10mm from the pulmonary/coumadin ridge – the structure between the left superior pulmonary vein and the LAA) [Freixa, 2021]
- coumadin ridge has often been misdiagnosed as a thrombus in the past
- left atrial appendages vary in size and shape
- according to registries, TEE at 45 days identified three groups of patients:
- with no leak
- with small leaks (< 5 mm) –
- with large leaks (≥ 5 mm)
- partial occlusion may create favorable conditions for thrombosis (Alkhouli, 2022)
- patients with large leaks (>3-5 mm) are usually kept on anticoagulation (if possible), or a vascular plug may be used
- patients with large leaks (>3-5 mm) are usually kept on anticoagulation (if possible), or a vascular plug may be used
- newer devices with improved sealing might reduce leak-related events
Air embolism
Pericardial tamponade
Occluder migration
Risk-benefit of the procedure
- given the safety profile of DOACs, the risk-benefit of the LAAO is uncertain
- unncessary withholding of anticoagulants may lead to undertreatment of the risk of stroke related to atrial cardiomyopathy
- after the procedure, antiplatelet therapy is often continued, which is itself risky in terms of bleeding risk [Mandrola, 2017]
- the bleeding risk of ASA vs. DOACs is not that different (see AVVEROES and RE-SPECT ESUS trials)
- in addition, many thrombi occur outside the LAA (due to diffuse atriopathy), and this risk increases with comorbidities [Mahajan, 2012]
- the bleeding risk of ASA vs. DOACs is not that different (see AVVEROES and RE-SPECT ESUS trials)
- on the other hand, observational data suggest that ischemic strokes in patients with Afib are less often disabling or fatal with LAAO than with DOAC prophylaxis (Turagam, 2024)