ADD-ONS / MEDICATION / ANTICOAGULANTS
Direct Oral Anticoagulants (DOACs)
Updated on 24/01/2024, published on 06/10/2022
- drugs, originally called novel oral anticoagulants (NOAC), are now more appropriately referred to as direct oral anticoagulants (DOAC)
- DOACs:
- are associated with the same or lower incidence of ischemic stroke and bleeding, especially intracerebral hemorrhage (ICH) compared to vitamin K antagonists (VKAs)
- on top of that, ICH occurring with DOACs has a better prognosis than ICH on VKAs [Inohara, 2018]
- have standardized dosing without the need for laboratory monitoring of the anticoagulant effect
- have relatively predictable pharmacokinetics
- have a rapid onset of action and resolution after discontinuation (facilitating easy switch or periprocedural discontinuation)
- are associated with the same or lower incidence of ischemic stroke and bleeding, especially intracerebral hemorrhage (ICH) compared to vitamin K antagonists (VKAs)
- data from RCTs have been confirmed in routine clinical practice (e.g., GLORIA-AF registry, etc.)
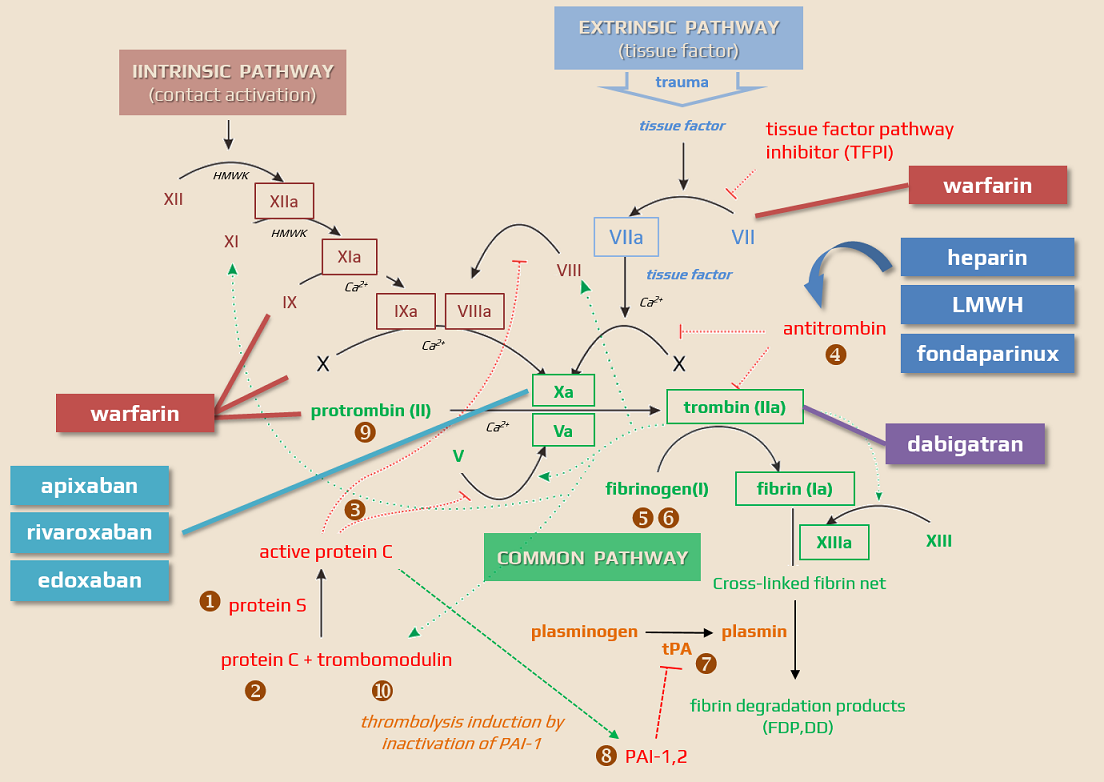
- specific antidotes are available for both dabigatran ( idarucizumab since 2016) and factor Xa inhibitors (andexanet alfa since 2019)
Indications for the use of direct oral anticoagulants
Stroke prevention in patients with atrial fibrillation
- in this indication, DOACs have long been preferred over VKAs in expert recommendations (EHRA 2018 I/A)
| Indications and contraindications for anticoagulants in patients with atrial fibrillation and associated cardiac comorbidities (ESC guidelines 2018) |
|
| nonvalvular atrial fibrillation |
|
| intracardiac thrombus |
|
|
mechanical heart valve |
|
| moderate to severe mitral valve stenosis |
|
| other valve defects, mild-moderate |
|
| severe aortic valve stenosis |
|
| bioprosthetic valve (>3 months since implantation) |
|
| hypertrophic cardiomyopathy (HCM) |
|
| transcatheter aortic valve implantation (TAVI) |
|
VTE treatment and prevention
- therapy and prevention of deep vein thrombosis (DVT) and/or pulmonary embolism (PE)
- for 3 months if the risk factor for thrombosis has passed (so-called secondary thrombosis after surgery, accident, or childbirth)
- for 6 months if no risk factor has been identified (idiopathic thrombosis)
- for 12 months in patients with significant thrombophilia, especially congenital thrombophilia, recurrent proximal phlebothrombosis, or symptomatic PE, and an increased risk of recurrence due to an acquired ongoing thrombophilic condition
- DOACs are not recommended in patients with a history of thrombosis diagnosed with antiphospholipid syndrome. Particular in patients with triple positivity (for lupus anticoagulants, anticardiolipin antibodies, and antibodies to beta 2-glycoprotein I), DOAC treatment may be associated with ↑ incidence of recurrent thrombotic events compared with warfarin
VTE prevention in knee and hip surgery
When are VKAs preferred over DOACs?
VKAs, such as warfarin, are preferred in certain clinical situations due to specific considerations and limitations of DOACs
- mechanical heart valve – DOACs are not recommended due to a higher risk of thromboembolic events and bleeding in this group
- AFib + moderate to severe mitral valve stenosis
- Afib + intracardiac thrombus (increased risk of stroke or systemic embolism with DOACs)
- Afib + hypertrophic cardiomyopathy (HCM) (limited data with DOACs)
- Afib + severe aortic valve stenosis (limited data with DOACs)
- severe renal impairment – e.g., creatinine clearance less than 15-30 ml/min, warfarin is often preferred because most DOACs are contraindicated or require cautious use
- antiphospholipid syndrome – particularly in patients with triple-positive antiphospholipid syndrome (lupus anticoagulant, anticardiolipin antibodies, and anti-beta-2-glycoprotein I antibodies), warfarin is favored as DOACs may be less effective and carry a higher risk of recurrent thrombotic events.
- pregnancy and lactation
- warfarin and DOACs are contraindicated during pregnancy due to teratogenic effects
- while DOACs are also generally avoided in lactation, warfarin may be a safe alternative postpartum
- extremes of body weight – warfarin might be preferred due to concerns about the efficacy and safety of DOACs in these populations
- drug interactions – if a patient is on medications that have significant interactions with DOACs, particularly those that affect the CYP3A4 and P-glycoprotein pathways, warfarin might be a safer choice
- cost considerations – in some regions, warfarin may be more cost-effective than DOACs
Overview of DOACs
| Direct thrombin inhibitors
Factor Xa inhibitors
|
| Content available only for logged-in subscribers (registration will be available soon) |
Pharmacokinetics and pharmacodynamics
DOACs are the substrate of two efflux pumps, P-gp and BCRP. The activity of both systems determines their bioavailability and the rate of bioelimination. Some DOACs (such as rivaroxaban and apixaban) are also substrates of CYP isoenzymes (mainly CYP3A4), which influence their transformation to inactive metabolites. Inhibition of P-gp increases bioavailability and slows elimination into the bile; blockade of CYP3A4 decreases inactivation and transformation. Many drugs are significant inhibitors of both CYP3A4 and P-gp. Clinically important inhibitors of both systems include azole-type antifungals (ketoconazole, fluconazole, itraconazole, etc.), antiretrovirals (e.g., ritonavir, etc.), and the macrolides clarithromycin and erythromycin.
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |||||
|
mechanism of action
|
direct thrombin inhibitor |
factor Xa inhibitor |
factor Xa inhibitor |
factor Xa inhibitor
|
||||
| time to peak |
1.5h |
2-4h |
2-4h |
1-2h |
||||
| bioavailability | 3-7% | 66%-100% (with food) |
> 50% | 62% | ||||
| prodrug | yes | no | no | no | ||||
| clearance extrarenal/renal | 20/80% | 65/35% | 72/28% | 50/50% | ||||
| CYP3/A4 | no | yes (elimination) | yes (elimination) | minimal | ||||
| plasma protein binding |
35% | 92-96% | 90% | 55% | ||||
| half-life | 12-17h | 5-9 h (younger) 11-13h (older) |
12h | 10-14h | ||||
| pharmacokinetic interactions |
P-gp PPIs |
CYP 3A4 P-gp |
CYP 3A4 P-gp |
P-gp (reduce dose by 50%) | ||||
| reversal |
idarucizumab (specific) | andexanet 4F-PCC (nonspecific) |
andexanet 4F-PCC (nonspecific) |
andexanet 4F-PCC (nonspecific) |
||||
| Content available only for logged-in subscribers (registration will be available soon) |
Interactions
Pharmacodynamic interactions
- concurrent use of other anticoagulants / antiplatelet drugs / NSAIDs ⇒ ↑ risk of bleeding
- carefully assess the clinical benefit of these combinations (a combination of DOAC + clopidogrel/ticagrelor is typically used in Afib patients after coronary stenting)
- consider prophylaxis using proton pump inhibitors (PPIs) in such cases
Pharmacokinetic interactions
- interactions between resorption, transformation, and elimination
- mainly via CYP3A4 and P-glycoprotein inhibitors (↑ effect up to 50%)
- azole antifungals (fluconazole, ketoconazole) and antiretrovirals (ritonavir, nelfinavir) are strong inhibitors of both CYP3A4 and P-gp
- inhibitors from the macrolide antibiotics group (clarithromycin and erythromycin)
- azole antifungals (fluconazole, ketoconazole) and antiretrovirals (ritonavir, nelfinavir) are strong inhibitors of both CYP3A4 and P-gp
- concomitant use of dronedarone and dabigatran is contraindicated; dose reduction is required when dabigatran is taken alongside verapamil
- decreased rivaroxaban levels have been reported with concomitant use of levetiracetam → see here
- except for dabigatran, there is no significant interaction between DOACs and antacids or PPIs [Bolek, 2017]
| Content available only for logged-in subscribers (registration will be available soon) |
Dosing
- rivaroxaban should be taken with food; other DOACs have no such limitation
| CrCl | ≥ 50 mL/min (0.83 mL/s) |
30-49 mL/min (0.5-0.82 mL/s) |
15-29 mL/min (0.25-0.49 mL/s) |
< 15mL/min (0.25 mL/s) |
| dabigatran (PRADAXA) |
2x 150 mg | 2x 110mg | ||
|
2x 110mg |
||||
| apixaban (ELIQUIS) |
2x 5 mg | 2x 2.5 mg | ||
| 2x 2.5mg if ≥ 2 of risk factors are present: age ≥ 80 let, creatinine > 133 umol/L, weight ≤ 60 kg |
||||
| rivaroxaban (XARELTO) |
1x 20 mg (with food) | 1x 15mg (with food) | ||
| edoxaban (LIXIANA) |
1x 60 mg | 1x 30mg | ||
|
1x 30mg |
||||
Typical errors that occur when using DOACs
| Content available only for logged-in subscribers (registration will be available soon) |
Dose reduction according to renal function
| Content available only for logged-in subscribers (registration will be available soon) |
Anticoagulant therapy switching
- switching antithrombotic medications must be carefully managed to ensure the patient’s safety and efficacy
- timing the initiation of the new medication to coincide with the next scheduled dose of the previous one is often recommended
Other specific situations in DOAC therapy
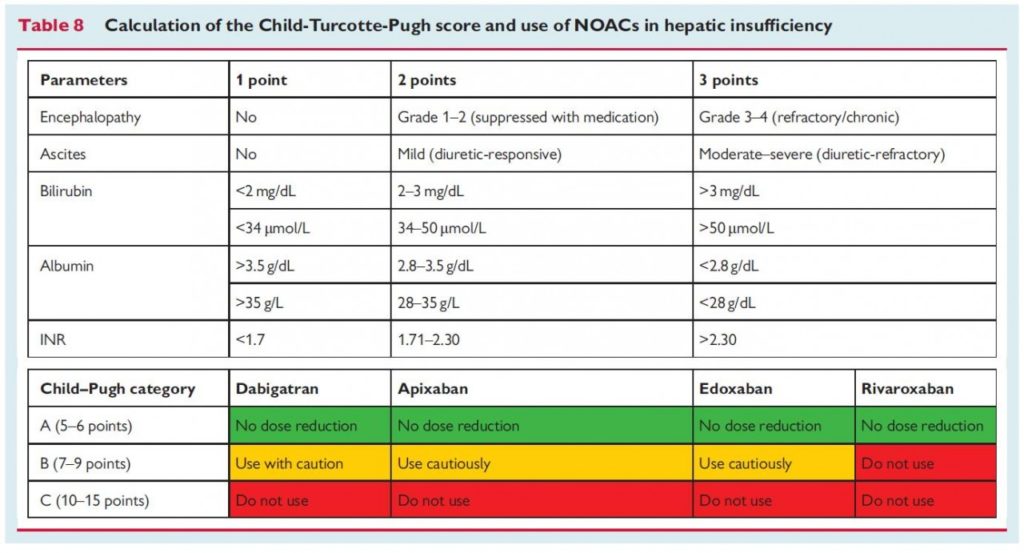
- advanced hepatopathy is associated with an increased bleeding risk but also with prothrombotic states
- additionally, liver disease may affect hepatic clearance and drug metabolism
- vitamin K antagonists (VKAs) are problematic due to spontaneously elevated INR values, making it difficult to choose an appropriate dose
- all DOACs are contraindicated in patients with liver disease associated with coagulopathy and clinically evident bleeding risk
- dabigatran, apixaban, and edoxaban may be used with caution in patients with Child-Pugh A and B cirrhosis – multidisciplinary management (including hepatologist and hematologist) is advisable
- rivaroxaban is contraindicated in both Child-Pugh B and C
- overweight and obesity (BMI > 25 and 30 kg/m2)
- no difference in efficacy and safety was found for apixaban or rivaroxaban in patients < 60 kg vs. > 60 kg or with a BMI ≥ 35 kg/m2
- due to limited data on extreme obesity, administration of VKAs may be considered in patients with BMI ≥ 40 kg/m2 or weight > 120-150) kg
- if DOAC treatment is required, specific laboratory monitoring is appropriate
- low body weight ( ≤ 50-60kg) – dose reduction is recommended
- DOAC concentration and bleeding risk may be increased; these patients often have comorbidities that may increase the risk of stroke and bleeding. Renal function may be overestimated in patients with low body weight
- dabigatran: dose reduction may be considered in patients with body weight ≤ 50 kg, but it is not necessary
- apixaban: dose reduction is recommended in patients with body weight ≤ 60 kg in combination with at least one other factor (age ≥ 80 years, blood clearance ≥ 133 μmol/L)
- rivaroxaban: no dose adjustment is required – equal efficacy and safety in patients < 70 kg vs. > 70 kg. Extremes in body weight (<50 kg or >120 kg) had little effect on rivaroxaban plasma concentrations (< 25% variation)
- edoxaban: dose reduction to 30mg is recommended in patients weighing ≤ 60 kg
- patients with Afib lasting ≥ 48 h (or of unknown duration) who are scheduled for electrical or pharmacologic cardioversion should be maintained on effective anticoagulation therapy for ≥ 3 weeks prior to the procedure or should undergo transesophageal echocardiography (TEE) to rule out intracardiac thrombosis
- anticoagulation therapy is required for at least 4 weeks after successful cardioversion; in high-risk patients, permanent anticoagulation should be continued
- fragility, advanced age, or dementia do not preclude anticoagulant therapy
- DOAC trials included a number of participants aged ≥ 75 years, and the use of DOACs resulted in a reduction in absolute risk compared to VKAs
- older patients are at risk of worsening renal function and also falls, which are often mistakenly considered as contraindication to anticoagulant therapy
- a Markov decision analytic model demonstrated that a patient on VKA would need to fall 295 times for the risk of subdural hematoma to outweigh the benefit of anticoagulation
- adherence to treatment is essential
- rule out pregnancy and recommend contraception before starting therapy
- DOACs may be associated with prolonged menstrual bleeding compared with VKA and LMWH
- DOACs are contraindicated during pregnancy and lactation
- atrial fibrillation is a common arrhythmia among athletes
- athletes taking oral anticoagulants for venous thromboembolism should avoid contact sports
- there is an increased risk of bleeding associated with injury during a seizure (with or without a fall)
- patients with generalized atonic seizures are particularly at risk of head injury
- generalized tonic seizures increase the risk of bleeding due to tongue injuries
- anticoagulants have numerous interactions with antiseizure medication
- in cases with severe interactions, DOACs should not be the preferred choice of anticoagulant drug
- tumors and atrial fibrillation are common in elderly patients; older age and malignancy are independent risk factors for both thrombosis and bleeding
- VTE – several meta-analyzes of small cohorts of patients with cancer in VTE studies have described equal or better efficacy of DOACs compared to VKA/LMWH
- atrial fibrillation – analysis of data in patients with active malignancy or history of malignancy in the ARISTOTLE trial showed superior efficacy and safety of apixaban compared to warfarin
- DOACs are probably preferred, but an interdisciplinary approach is advisable
- given the short duration of action (12-24 hours), noncompliance with DOACs is associated with a higher thromboembolic risk compared to VKA
- consider switching to VKA in these patients, as missing one dose of VKA will not significantly decrease anticoagulant activity
Monitoring
Discontinuation of DOACs before surgery
- DOACs, compared to warfarin, have predictable Cmax and T1/2
- consider:
- age + weight
- renal function
- history of bleeding
- concomitant medication and comorbidities
- specific bleeding risk associated with the procedure
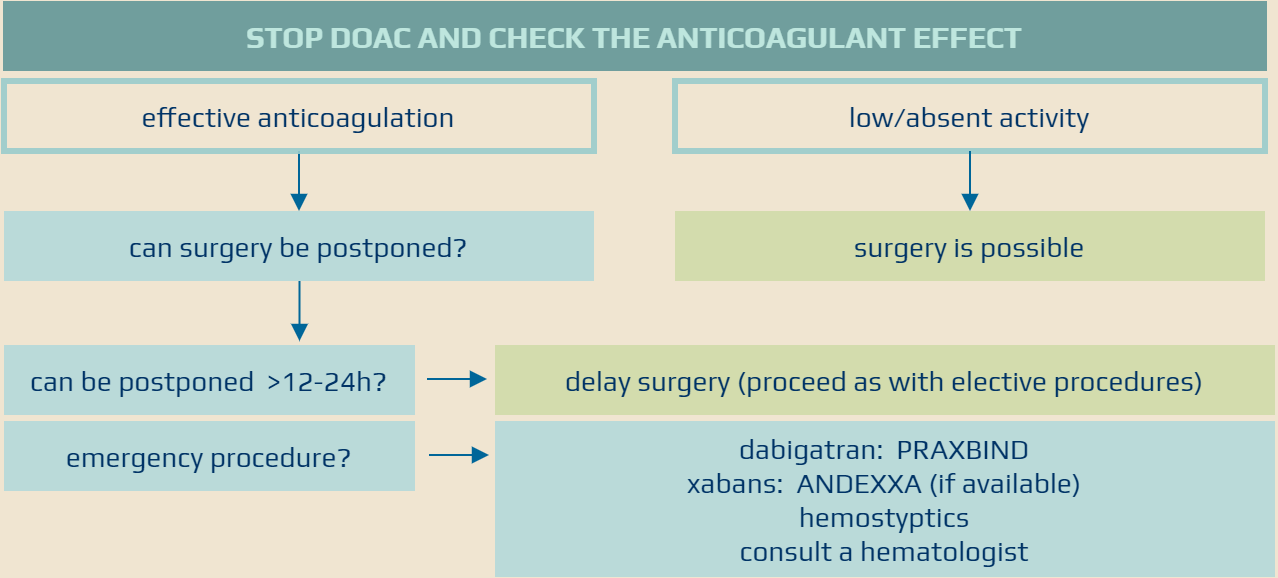
Elective surgery
- check:
- serum chemistry panel, including renal function
- complete blood count (CBC)
- coagulation tests, incl. specific tests corresponding to the particular drug
- for detecting the anticoagulant effect or other hemostatic disorders (thrombocytopenia, etc.)
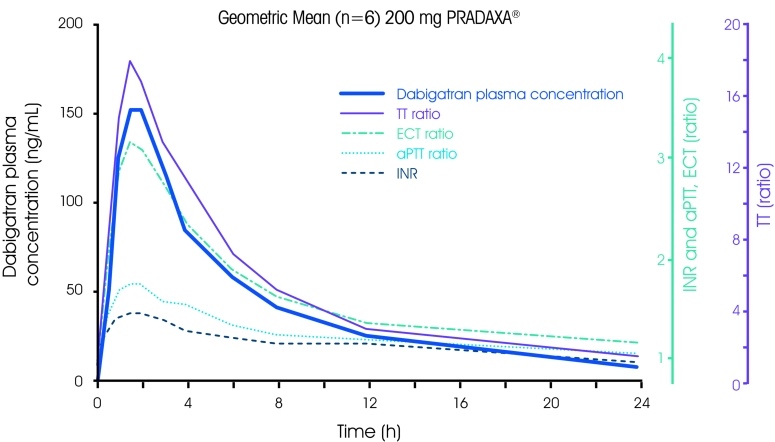
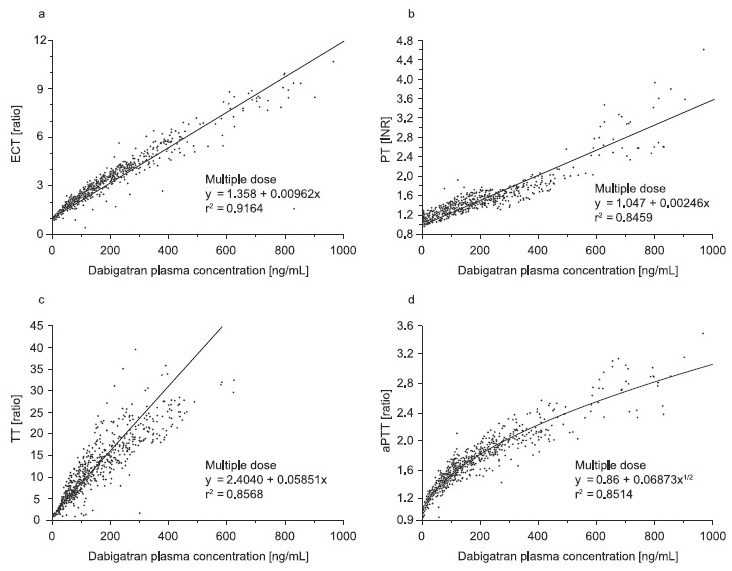
- → DOAC monitoring
- if needed, prefer specific antidotes:
- idarucizumab for patients using dabigatran
- andexanet alfa for patients using xabans (problematic availability and price)
- if a specific antidote is not applicable, consider substitution therapy (prothrombin complex concentrate, fresh frozen plasma)
- consider delaying surgery until APTT/anti-Xa levels normalize; consult a hematologist if urgent surgery is required
Regional anesthesia
DOAC and recanalization therapy
- stroke can occur even in patients who are on anticoagulation therapy
- there is no contraindication to mechanical recanalization
- follow specific protocols when considering intravenous thrombolysis (IVT)