MEDICATION
Switching of antithrombotic therapy
Updated on 14/02/2024, published on 24/01/2024
- switching antithrombotic medications must be carefully managed to ensure patient’s safety and efficacy
- factors such as the type of antithrombotic medication, its indication, the patient’s medical history, and the reason for switching are crucial in determining the appropriate strategy
- common reasons for switching include perioperative management, development of contraindications to DOACs, or the need for a shorter-acting or parenteral anticoagulant
- in general, a new drug is started when the next scheduled dose of the previous drug is due, with exceptions in cases where higher plasma concentrations are expected, such as in patients with renal insufficiency
- close monitoring and evaluation of the patient’s clinical status and laboratory results are essential during this transition to minimize the risk of both thrombotic and bleeding complications
- start VKA while continuing LMWH (see notes on bridging)
- treatment usually starts with a dose of 1.5-5 mg/day of warfarin to allow a gradual onset of effect
- high initial doses may lead to a rapid depletion of protein C and S ⇒ ↑ risk of hypercoagulable state with warfarin-induced skin necrosis
- monitor INR regularly
- discontinue LMWH if INR >2 for at least 24 hours
- adjust VKA dose based on measured and target INR values
- stop VKA and confirm INR is below therapeutic range
- LMWH should be started once INR is < 2
- adjust LMWH dose based on renal function and weight
- monitor for efficacy and safety
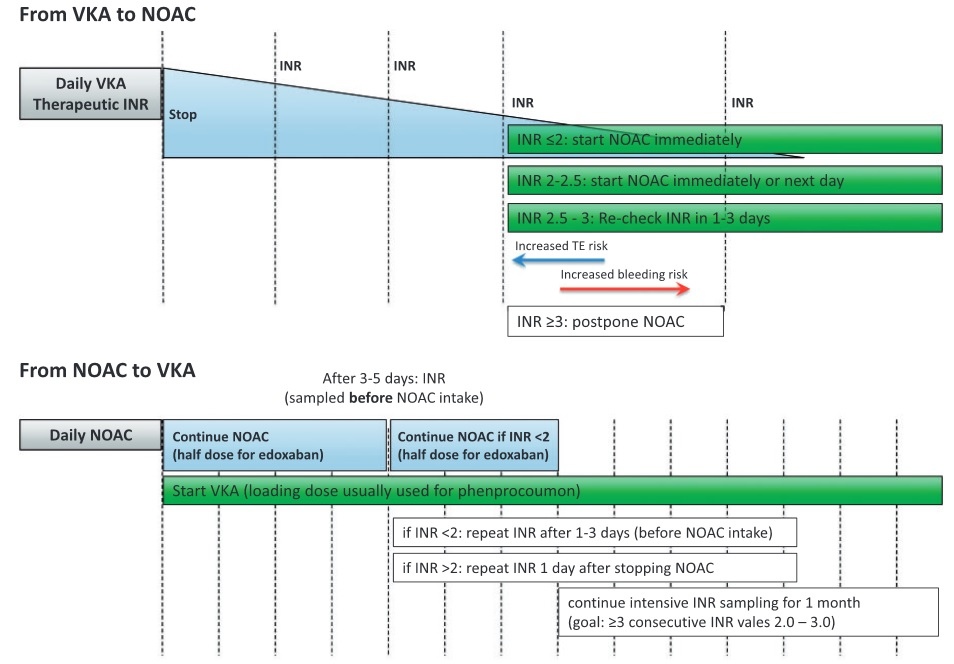
- all DOACs can be started immediately if the INR < 2
- official recommendation:
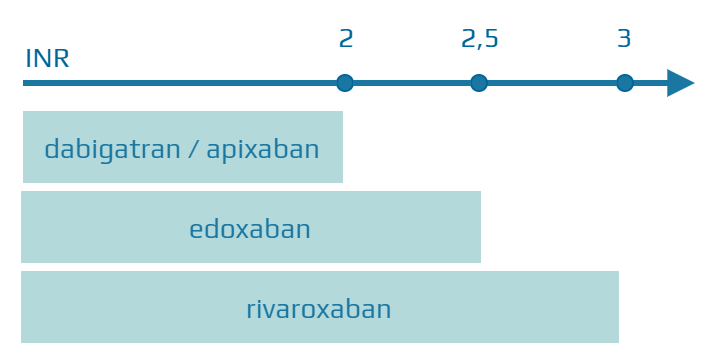
- rivaroxaban – INR < 3
- edoxaban – INR ≤ 2.5
- apixaban/dabigatran – INR < 2
- if the INR is > 2.5, check the INR the next day, or you can start using rivaroxaban if the INR < 3
- once starting with rivaroxaban, apixaban, or dabigatran, INR measurement becomes unreliable and may be falsely elevated
- DOAC and VKA (vitamin K antagonist) can be administered simultaneously until INR is >2
- DOACs may affect INR (falsely elevated values) – always measure INR before taking another DOAC during concomitant administration and measure again 24-48h after discontinuing DOACs
- it is recommended to reduce the dose when starting VKAs while using edoxaban
- for patients taking the 60 mg dose, administer edoxaban at 30 mg once daily along with an appropriate dose of a VKA
- for patients taking the 30 mg dose, administer edoxaban at 15 mg once daily with a proper dose of a VKA
- close monitoring of INR is recommended during the first month until stable INR values are achieved
- if intermediate concomitant DOAC + VKA is not applicable, consider switching from DOAC to LMWH and then from LMWH to VKA (especially in patients at high risk of thromboembolism)
- no need for overlap or bridging
- from heparin: start DOAC 2 hours after discontinuing IV heparin
- from LMWH: DOACs should be administered at the time of the next scheduled LMWH dose (some authors recommend starting DOACs 2 hours earlier)
- in patients with renal insufficiency, beware of prolonged LMWH elimination
- calculate the appropriate dose of LMWH based on the patient’s weight and indication.
- heparin/LMWH can be started at the time of the next scheduled DOAC dose
- consider checking anti-Xa levels for LMWH to ensure therapeutic anticoagulation
- if switching to IV UFH, do not start with a heparin bolus → unfractionated IV heparin protocol
- start the new DOAC at the time when the next dose of the original DOAC was scheduled to be given
- except in situations where a higher than therapeutic plasma concentration is expected (e.g., in patients with renal insufficiency)
- start DOACs immediately and discontinue antiplatelet therapy (SAPT or DAPT) unless combination therapy is indicated (recent stenting + AFib, etc.)
- short overlap of both drugs poses a very low risk
- discontinue DOACs and start antiplatelet therapy immediately (antiplatelets needs time to reach full effect)
- a short overlap of DOAC and antiplatelet effects is associated with relatively low bleeding risk (AFib patients with recent stenting safely receive prolonged combination of DOAC+antiplatelet drug)
LMWH bridging
- bridging anticoagulation involves the administration of a short-acting anticoagulant (usually LMWH) in the following situations:
- initiation of anticoagulant therapy
- periprocedural bridging → see separate chapter