ISCHEMIC STROKE / CLASSIFICATION AND ETIOLOGY
Moyamoya disease
Updated on 10/06/2024, published on 11/05/2023
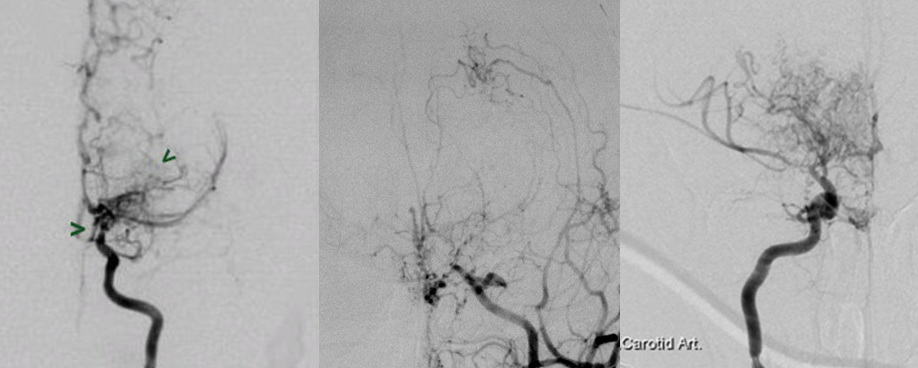
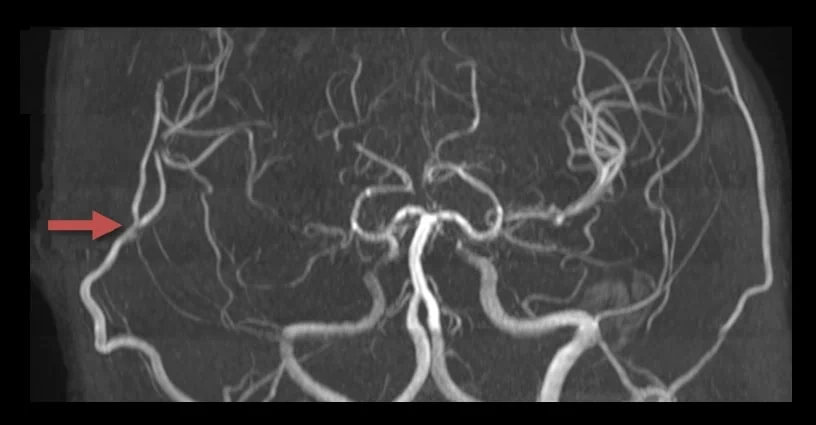
- progressive stenoocclusive disorder affecting the terminal segment of the internal carotid artery (ICA) and proximal segments of the arteries forming the circle of Willis (ACA, MCA, PCA)
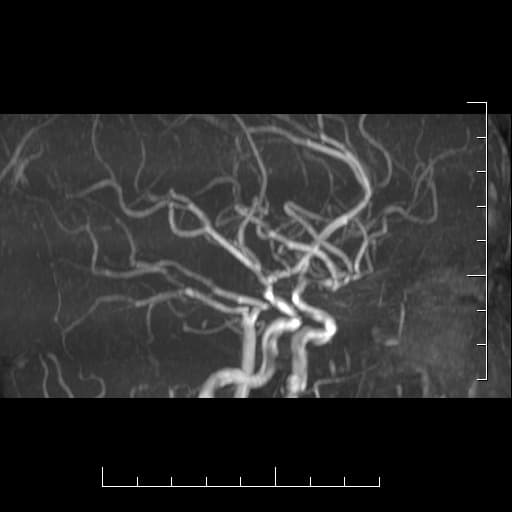
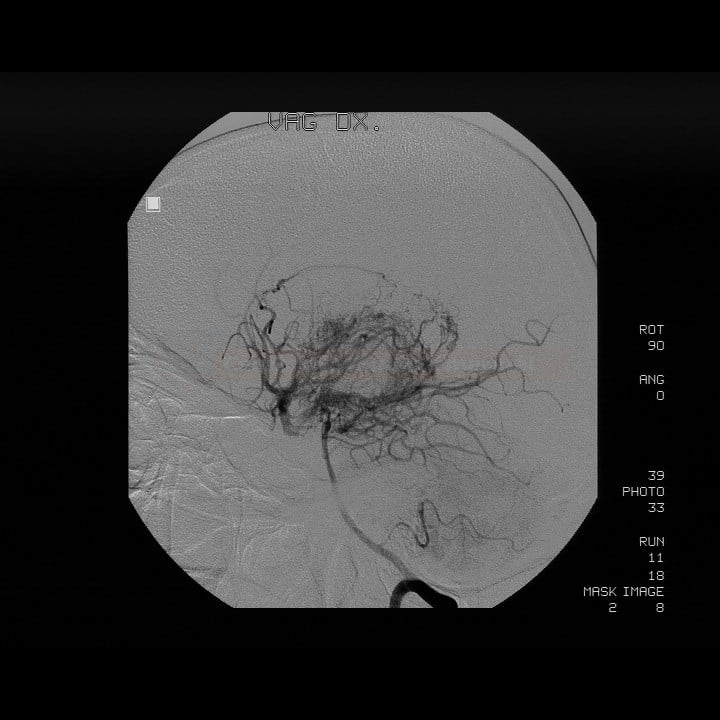
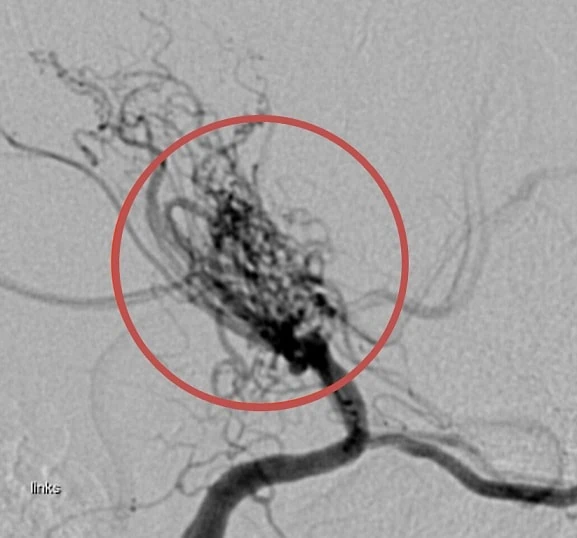
- an abnormal network of fine collateral arteries (lenticulostriate arteries, thalamic perforators, and pial arteries) is formed near stenotic vessels, resembling smoke puffs (moya-moya)
- the process is usually bilateral, but unilateral manifestation does not exclude the diagnosis
Etiology
- moyamoya angiopathy (moyamoya pattern) may be caused by a variety of congenital and acquired diseases (see table)
- proliferation and thickening of the intima of the terminal ICA segment and proximal MCA segment predominate; the proliferating intima may contain lipid deposits, but there is no evidence of inflammation [Bang, 2015]
- thrombi may be present
- associated neovascularization occurs as a compensatory mechanism (collateral circulation)
- in practice, moyamoya should be considered more as a radiologic entity that can be associated with several diseases
- acquired (e.g., moyamoya syndrome in vasculitis) – see table
- congenital – sporadic and familial forms (10% are reported, but the number is probably higher)
- AD transmission with low penetrance has been demonstrated in familial cases of MMD with linkage to chromosomes 3,6,8,12 and 17 (e.g., 17q25.3 Raptor); Ring finger 213 (RNF213) appears to be an important gene, its exact function is unknown [Bang, 2015]
- the moyamoya pattern may be caused by other inherited vasculopathies (Grange syndrome, ACTA2 mutations, etc.)
| Moyamoya disease |
|
| Moyamoya syndrome – acquired conditions |
|
Epidemiology
- predominantly in Asians, with the highest prevalence in Japan (3.16 cases/100,000 inhabitants)
- rarely affects Caucasians, African Americans, and Hispanics
- peak incidence around age 4 (2/3) and then at age 30-40 (1/3)
- approx. 10% of cases are familial (inherited)
Clinical presentation
- onset of symptoms occurs across a wide age range (4 months to 67 years), peaking in the 1st and 3-4th decade of life
- the clinical course is variable, ranging from asymptomatic forms to TIAs or strokes with permanent neurological deficit
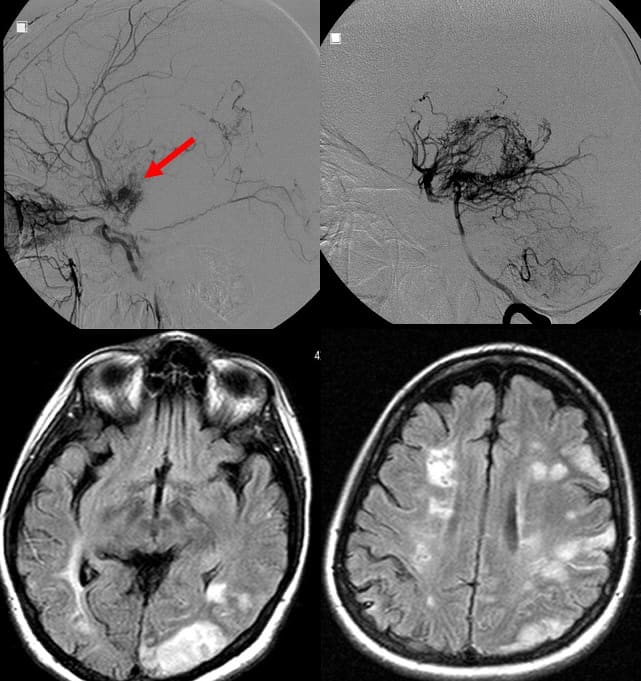
- in children, common symptoms include mono- or hemiparesis, sensory disturbances, involuntary movements, headache, cognitive impairment, and epileptic seizures; intracerebral bleeding is relatively rare
- in adults, intraventricular, subarachnoid, or intraparenchymal bleeding is more common (up to 30% of cases)
- hematomas most commonly result from ruptured collaterals [Kim, 2017]
- watershed infarcts are common
- clinical presentation, especially the incidence of bleeding, is influenced by racial factors, with a higher prevalence of bleeding observed in Asians compared to Caucasians
Diagnostic evaluation
Diagnostic criteria
| Radiological and histological diagnostic criteria for MMD |
|
| Angiographic findings suggesting the diagnosis of moyamoya | |
|
|
|
Histopathologic findings suggesting the diagnosis of moyamoya
|
|
|
|
Moyamoya disease staging [Suzuki 1969]
|
|
|
Stage 1 – narrowing of the carotid bifurcation
Stage 2 – formation of collaterals (“moyamoya arteries”), stenosis of the terminal ICA, dilatation of the MCA and ACA
Stage 3 – intensive formation of moyamoya arteries, progression of stenoses of ICA, MCA, and ACA
Stage 4 – gradual disappearance of moyamoya arteries, the disappearance of PCA, further narrowing of ICA, MCA, and ACA
Stage 5 – a further reduction of moyamoya arteries with occlusion of ICA, ACA, and MCA
Stage 6 – ICA essentially disappears; the brain is supplied by the ECA
|
- the staging system, initially described by Suzuki and Takaku in 1969, is still used
- staging is based on DSA findings, although a similar system may be applied to MRA or CTA
- disease progression is more rapid in children compared to adolescents or adults
Imaging modalities
- digital subtraction angiography (DSA) – the gold standard, practically replaced by MRA/CTA
- MR+MRA / CT+CTA
- parenchymal lesions
- typically watershed or territorial infarcts
- hemorrhages or asymptomatic microbleeds (GRE/SWI sequences)
- cerebral atrophy
- evidence of stenoses in typical locations (distal ICA, M1, or PCA) + a characteristic collateral network
- unilateral finding is observed in up to 18%
- vessel wall imaging can help in DDx (from atherosclerosis or inflammation)
- concentric stenosis
- minimal or absent enhancement
- homogeneous T2 signal
- absence of atherosclerotic changes
- detection of collaterals
- perforator network (puff of smoke) is mainly visualized on DSA (to a lesser extent on CTA or MRA)
- Ivy sign – increased signal intensity on MR FLAIR and T1C+ in the leptomeninges and perivascular spaces – indicates slow or retrograde flow in the leptomeningeal and cortical arteries
[Ohta, 1995] [Tharayil, 2019]
- parenchymal lesions
- neurosonology
- the flow in the affected and distal segments + vasomotor reactivity (CVR) may be monitored ⇒ may help to indicate surgical treatment
Genetic testing
- mutations testing in the RNF213 gene located on 17q25 (in Asians) → see here
- experts suggest against systematic variant screening of RNF213 (ESO guidelines, 2023)
- “moyamoya panel” in the differential diagnosis (incl. ACTA2, Grange sy, etc.)
Hemodynamic assessement
- hemodynamic assessment (by CT, MRI, SPECT, PET, and ultrasound) is suggested for all patients with MMA (ESO guidelines, 2023, consensus)
- imaging methods most familiar and available, depending on individual institutions, should be used
Differential diagnosis
- moyamoya syndrome (see the table above) must be distinguished
Management
Surgery
- always consider the risk/benefit when evaluating treatment options
- surgical treatment is mainly indicated in patients with a progressive, symptomatic course
- the primary aim is to improve flow and prevent further formation of fragile collaterals
- surgical treatment is mainly indicated in patients with a progressive, symptomatic course
- no clinical study comparing the effect of conservative and surgical treatment is available
- the JAM trial showed marginal benefit in preventing rebleeding
- several small-scale studies have produced conflicting results
- revascularization recommendations (ESO 2023):
- adult patients:
- adult patients with hemorrhagic presentation: surgery (evidence only for direct STA-MCA bypass) in case of cerebral hemodynamic impairment
- adult patients with ischemic presentation: revascularization should be considered in case of clinical symptoms and/or imaging markers of hemodynamic impairment (uncertain risk/benefit)
- asymptomatic adult patients: consider conservative treatment except in patients with both cerebral hemodynamic impairment and silent ischaemic lesions
- pediatric patients:
- suggest revascularization surgery if there is evidence of ongoing ischemic symptoms or cerebral hemodynamic impairment; except in case of large territorial ischemic lesion
- there is continuous uncertainty over the risks and benefits of cerebral revascularization
- adult patients:
- surgical revascularization should be performed in a referral center and by a neurosurgeon with significant experience in surgical revascularization techniques
- according to a meta-analysis, better outcomes can be expected with direct and combined anastomosis compared to indirect anastomosis alone (Nguyen, 2022)
- direct/combined revascularization is suggested instead of indirect strategies for reducing the risk of stroke (ESO guidelines, 2023)
- in pediatric patients, a combined revascularization instead of indirect strategies is suggested whenever technically possible to decrease the short-term risk of stroke
- moya-moya syndrome with potential causal treatment (e.g., vasculitis) should be excluded before proceeding with surgery [Fujimura, 2015]
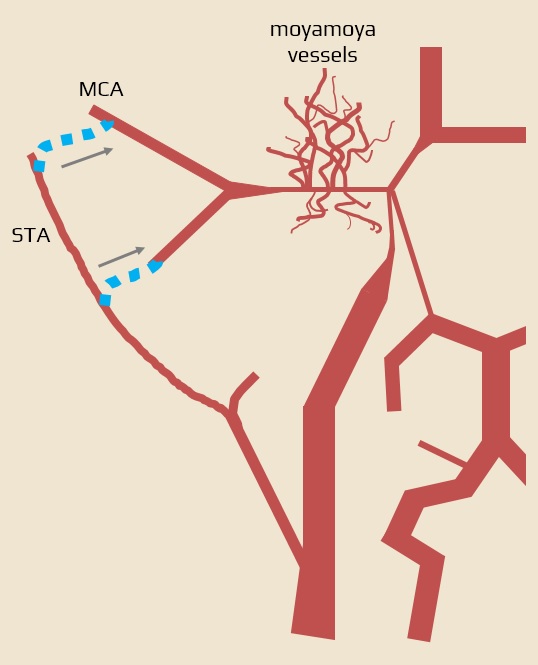
- direct anastomosis between the superficial temporal artery (STA) and the middle cerebral artery (MCA) – STA-MCA anastomosis [Golby, 1999]
- vessel-to-vessel anastomosis offers immediate revascularization (convenient when symptoms are progressive)
- a recipient vessel size of > 1-1.5 mm is required
- problematic in pediatric cases
- indirect anastomosis (flow improvement can be expected within weeks because a new capillary network is built; feasible in children)
- encephalo-duro-arterio-synangiosis (EDAS)
- encephalo-duro-arterio-myo-synangiosis (EDAMS)
- pial synangiosis
- combined anastomosis (direct + indirect)
Conservative therapy
- there is no specific drug therapy to alter the course of the disease
- standard treatment protocols for ischemic and hemorrhagic strokes are applied
- efficacy and safety of thrombolysis is unclear; increased risk of bleeding from fragile collaterals needs to be considered
- efficacy and safety of thrombolysis is unclear; increased risk of bleeding from fragile collaterals needs to be considered
- for secondary stroke prevention, antiplatelet therapy is commonly used
- not an evidence-based approach; should be individualized, taking into account the type and severity of the stroke, angiographic findings, and a risk-benefit analysis of the therapy
- antiplatelet therapy is also accepted by the AHA/ASA Guidelines 2021 (2b/C-LD) and ESO guidelines 2023 (expert opinion)
- bleeding is common in Asians, complicating the use of antithrombotic agents
- not an evidence-based approach; should be individualized, taking into account the type and severity of the stroke, angiographic findings, and a risk-benefit analysis of the therapy
- the role of antiplatelet therapy in primary stroke prevention is unclear
- long term ASA is suggested (ESO guidelines 2023, expert opinion)
Prognosis
- prognostic factors
- rate and extent of vascular occlusions
- patient’s ability to form functional collateral circulation
- age
- neurologic deficit severity
- extent of cerebral infarction
- some patients remain stable without surgical intervention; sometimes, they stabilize after severe cerebral infarction or hemorrhage with a permanent disability
- approx. 50-60% of patients exhibit cognitive impairment due to multiinfarct dementia
- mortality rate ~10% in adults and ~4-5% in pediatric patients
- the most common cause of death is intracranial hemorrhage

![Enlarged intima (black arrow) and intraluminal thrombus (blue arrow) in moyamoya [Scott, 2009]. Enlarged intima (black arrow) and intraluminal thrombus (blue arrow) in moyamoya [Scott, 2009]](https://www.stroke-manual.com/wp-content/uploads/2023/05/moyamoya_histology.jpg)