ISCHEMIC STROKE / ACUTE THERAPY
Intra-arterial thrombolysis
Updated on 03/01/2024, published on 16/09/2022
- intra-arterial thrombolysis (IAT) involves the administration of a thrombolytic agent via catheter directly into the occluded artery
- several trials using prourokinase (MELT, PROACT) and alteplase ( IMS-3, SYNTHESIS) showed the benefit of this therapy
- MELT The Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial)
- PROACT-2 (Prolyse in Acute Cerebral Thromboembolism)
- IMS 3 (Interventional Management of Stroke trial)
- demonstrated that the combined IV+IA approach to recanalization may be more effective than standard IVT alone for moderate to severe strokes, with a similar safety profile
- alteplase (tPA) is used in clinical practice
- the dosage is not standardized
- the standard therapeutic time window for IAT was 0-6h; studies with advanced imaging (showing penumbra) are not available (the individual window can theoretically be derived from IVT studies – but this is not discussed in the guidelines)
Indications
- indications are the same as for mechanical thrombectomy (MT)
- IAT is now rarely used as a stand-alone procedure; MT is preferred as a more potent recanalization technique
- IAT can be performed:
- when the thrombus is inaccessible for mechanical recanalization (extreme kinking, etc.)
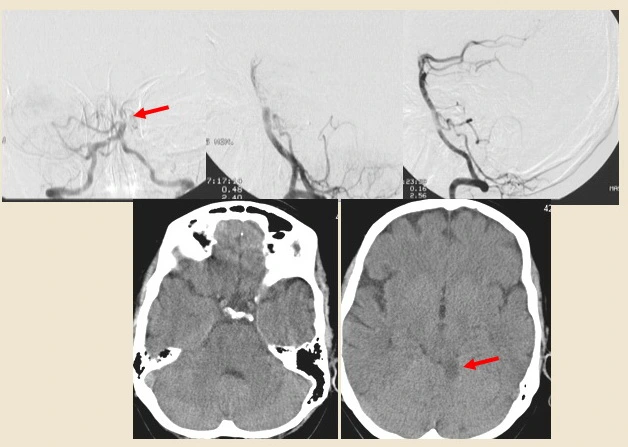
- to treat distal embolization (incl. those occurring during embolectomy) (Khatri, 2022) [Kaesmacher, 2019]
- as a rescue procedure after failed MT; the efficacy of MT followed by IAT was reported in the CHOICE trial (Zaidi,2019)
CHOICE trial
- The CHOICE trial was a Phase 2b randomized, double-blind, placebo-controlled trial conducted from December 2018 through May 2021 in seven stroke centers in Catalonia, Spain
- It aimed to investigate whether adjunct intra-arterial alteplase after thrombectomy improves outcomes following reperfusion in patients with large vessel occlusion acute ischemic stroke
- the trial included 121 patients treated with thrombectomy within 24 hours after stroke onset. Participants were randomized to receive intra-arterial alteplase (0.225 mg/kg; maximum dose, 22.5 mg) infused over 15-30 minutes (61 patients) or placebo (52 patients)
- the primary outcome was the difference in the proportion of patients achieving a score of 0 or 1 on the 90-day modified Rankin Scale. Results showed that 59.0% of participants with alteplase and 40.4% with placebo achieved this score
- the study was terminated early due to the COVID-19 pandemic, affecting placebo availability and enrollment rate
- these findings should be interpreted as preliminary and require replication due to study limitations
Contraindications
Apart from the usual contraindications to thrombolysis :
- arterial dissection
- proximal (usually carotid) stenosis or significant coiling preventing microcatheter delivery to the occluded segment
- extensive signs of ischemia on CT or absence of penumbra on CT perfusion
Procedure
Dosing according to original IAT protocols
- dilute 20 mg with 50mL NS (solution: 1mL=0.4mg tPA)
- the dosage is not standardized; most commonly in the range of 22-69 mg [Qureshi, 2000]
- IAT after the previous IVT
- usually, 20mg of tPA is administered IA within 1 hour
- initial bolus 5mg followed by 45mL/h infusion
- control super-selective angiograms are performed every 15 minutes until thrombus dissolution or until a maximum total dose of 20mg is reached
- the combination of full-dose IVT followed by thrombectomy or IAT at a dose of 20mg given within 1 hour appears to be safe [Hashem, 2007]
- initial bolus 5mg followed by 45mL/h infusion
- IAT with a dose of 69mg has also been reported to be safe after previous IVT [Hassan, 2012]
- usually, 20mg of tPA is administered IA within 1 hour
- IAT without previous IVT
- usually, 40 mg of tPA is administered over 2 hours
- initial bolus 5ml, followed by 45mL/h infusion in the first hour and 50 mL/h in the second hour
- control super-selective angiograms are performed every 15 minutes until thrombus dissolution or until a maximum total dose of 40mg is reached
- initial bolus 5ml, followed by 45mL/h infusion in the first hour and 50 mL/h in the second hour
- in the SYNTHESIS trial, even a dose of 0.9 mg/kg was administered (without prior IVT)
- in the CHOICE trial, a dose of 0.225 mg/kg was used; a maximum dose of 22.5 mg, administered as a 15-30 minute infusion
- usually, 40 mg of tPA is administered over 2 hours
- if recanalization is achieved, wait 15-20 minutes and perform a control angiogram to rule out early reocclusion
IAT after previous mechanical thrombectomy
- tPA dosing according to the CHOICE trial – 0.225 mg/kg; max 22.5 mg, administered within 15-30 minutes in patients with TICI 2b-3