ISCHEMIC STROKE / CLASSIFICATION AND ETIOPATHOGENESIS
Stroke and celiac disease
Updated on 07/11/2023, published on 16/02/2023
Introduction
- celiac disease (also known as gluten enteropathy, endemic sprue, or primary malabsorption syndrome) is an immune-mediated systemic disease triggered by gluten in genetically susceptible individuals
- this response results in characteristic damage to the villi, leading to malabsorption
- it is characterized by a variable combination of clinical symptoms in the presence of specific antibodies, HLA-DQ2 or HLA-DQ8 haplotypes, and enteropathy
- heterogeneous clinical signs and symptoms can manifest in either childhood or adulthood; the disease may also be asymptomatic
- celiac disease is associated with an increased incidence of cardiovascular diseases, including stroke (OR 1.4) [El Moutawakil, 2009] [Fabbri, 2012] [Medscape, 2014]
- stroke may occur without clinical signs of malabsorption (both in adulthood and childhood)
- gluten intolerance is lifelong and incurable, but symptoms resolve with adherence to a gluten-free diet
Stroke and celiac disease
- celiac disease is associated with an increased risk of venous and arterial thromboembolism (HR for stroke being 1.45) [Poulin, 2015]
- exact relationship between celiac disease and stroke and its clinical significance remain areas of ongoing research
- young patients with cryptogenic stroke might be evaluated for celiac disease (especially if they have other suggestive symptoms or family history)
- possible mechanisms of vascular involvement:
- the chronic inflammatory state may contribute to endothelial dysfunction and a prothrombotic state
- malabsorption in celiac disease can lead to deficiencies in essential vitamins and nutrients, such as vitamin B12, which may be linked to hyperhomocysteinemia, a potential risk factor for stroke
- coagulation abnormalities
- increased risk of other cardiovascular diseases, which could indirectly increase the risk of ischemic stroke
- CNS vasculitis
- diet reduces the risk of associated complications, including vascular events
Clinical presentation
- when food containing gluten is consumed, the lining of the small intestine becomes inflamed, and the epithelial cells of the intestine are damaged
- as a result, nutrients may be difficult to absorb and remain undigested in the intestine
- symptoms:
- weight loss
- diarrhea
- vomiting, loss of appetite, fatigue
- neurological symptoms (epilepsy, bilateral occipital calcification, cerebellar ataxia, degenerative central nervous system disease, peripheric neuropathy, myopathy, and stroke)
- neurological symptoms may occur even without prior signs of malabsorption. !!
- celiac disease is associated with an increased risk of:
- non-Hodgkin’s lymphoma
- diabetes
- Hashimoto’s thyroiditis
- dermatitis herpetiformis Duhring
- dilated cardiomyopathy (increased incidence of CD in patients with idiopathic dilated cardiomyopathy as well as in patients with secondary cardiomyopathy has been reported recently) (Frustaci, 2002)
Diagnostic evaluation
Antibodies detection
- test antibodies against tissue transglutaminase (anti-TG2 or anti-tTG) and endomysium (EMA) in blood, or antibodies against deamidated gliadin peptides (DGP)
- tTG is an enzyme that modifies gluten peptides, making them more immunogenic. In celiac disease, the immune system mistakenly targets and attacks this enzyme, leading to the production of anti-tTG antibodies
- the EMA test is highly specific for celiac disease but is more labor-intensive
- the DGP test may be useful in cases where traditional antibody tests are unclear, especially in children < 2 years of age
- anti-gliadin antibodies (AGA) have been abandoned due to their low specificity
- initially, test for total IgA and anti-TG2 in the IgA class
- the test is important because some people (∼ 2-3% of celiac patients) are deficient in IgA, which can lead to false-negative results in the IgA-tTG and IgA-EMA tests
- in the presence of IgA deficiency (< 0.2 g/L), test for EMA and anti-TG2 in the IgG class (or anti-DGP antibodies)
- a decline in antibody levels typically indicates that the patient is adhering to a gluten-free diet and that the intestine is healing
HLA identification (Human Leukocyte Antigen)
- HLA-DQ2 (found in 95% of patients) and HLA-DQ8 (5% of patients) are strongly associated with celiac disease
- their presence alone does not confirm the disease – HLA-DQ2 or HLA-DQ8 positivity is very common (25-40% of the population)) and only a fraction of these individuals develop celiac disease (these HLA types are necessary for the disease’s development but not sufficient on their own)
- HLA-DQ2 and HLA-DQ8 negativity virtually excludes the diagnosis of celiac disease
- first-degree relatives of individuals with celiac disease are at an increased risk of developing the disease; in these individuals, HLA typing may be a useful screening tool
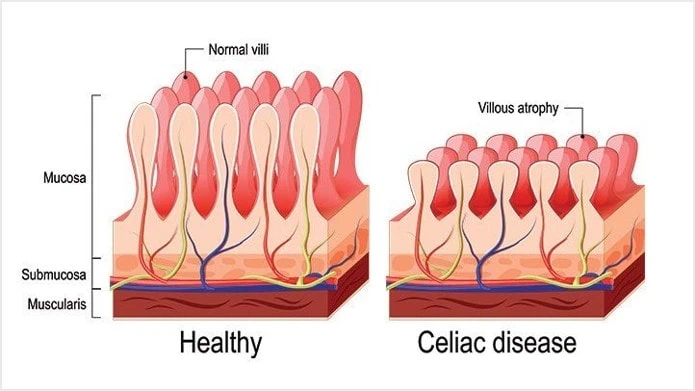
Biopsy
- analysis of duodenal biopsy is the gold standard, especially in elderly patients
- typical finding includes villous atrophy with hyperplasia of the crypts and an increased intraepithelial lymphocyte count
All tests should be performed before the patient starts a gluten-free diet
The indicative blood test for the presence of IgA transglutaminase antibodies can be done at home (tests are available over the counter). However, it should not replace a professional medical evaluation.
Management
- a gluten-free diet is crucial; with adherence to the diet, the intestine heals, reducing the risk of long-term complications
- patients should be informed about potential vascular risks and should be managed for traditional vascular risk factors
- if vasculitis is confirmed, additional treatments like immunosuppressive drugs might be considered based on the severity and the patient’s clinical presentation