ADD-ONS / MEDICATION / ANTICOAGULANTS
Dabigatran (Pradaxa)
Updated on 24/01/2024, published on 19/10/2022
Dabigatran (PRADAXA) is an oral anticoagulant drug used to treat and prevent venous thromboembolism (VTE) and stroke in people with nonvalvular atrial fibrillation (NVAF) through direct thrombin inhibition. It is used as an alternative to warfarin, which does not require laboratory monitoring or dietary restrictions. Dabigatran belongs to a group of drugs known as direct oral anticoagulants (DOACs)
Indication
- Primary and secondary stroke prevention in non-valvular atrial fibrillation (NVAF) – RE-LY trial
- Treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) (RECOVER, RECOVER II, RE-SONATE, RE-MEDY trials)
- VTE prevention in the knee and hip surgery
Contraindications
- hypersensitivity to the medicinal substance or any of the excipients
- pregnancy and breastfeeding
- severe renal impairment → Cockcroft-Gault formula!
- adults: creatinine clearance <30 mL/min (0.5 mL/s)
- pediatric patients: creatinine clearance <50 mL/min/1.72 m2
- clinically significant active bleeding
- lesions or conditions with a significant risk for major bleeding
- current or recent gastrointestinal ulcerations, presence of malignant tumors
- recent brain or spinal injury, a recent surgical procedure in the brain, spine, or eye
- recent intracranial hemorrhage, known presence or suspected esophageal varices, arteriovenous malformations, vascular aneurysms, or severe
intraspinal or intracerebral vascular anomalies
- spontaneous or pharmacological (concomitant antithrombotic drugs) disorder of hemostasis
- liver disease – all DOACs are contraindicated in patients with liver disease associated with coagulopathy and clinically apparent risk of bleeding
- concomitant therapy (especially potent P-glycoprotein inhibitors – see interactions below):
- dronedarone (Multaq)
- rifampicin
- ketoconazole, itraconazole
- cyclosporine
- tacrolimus
- mechanical heart valves requiring VKAs
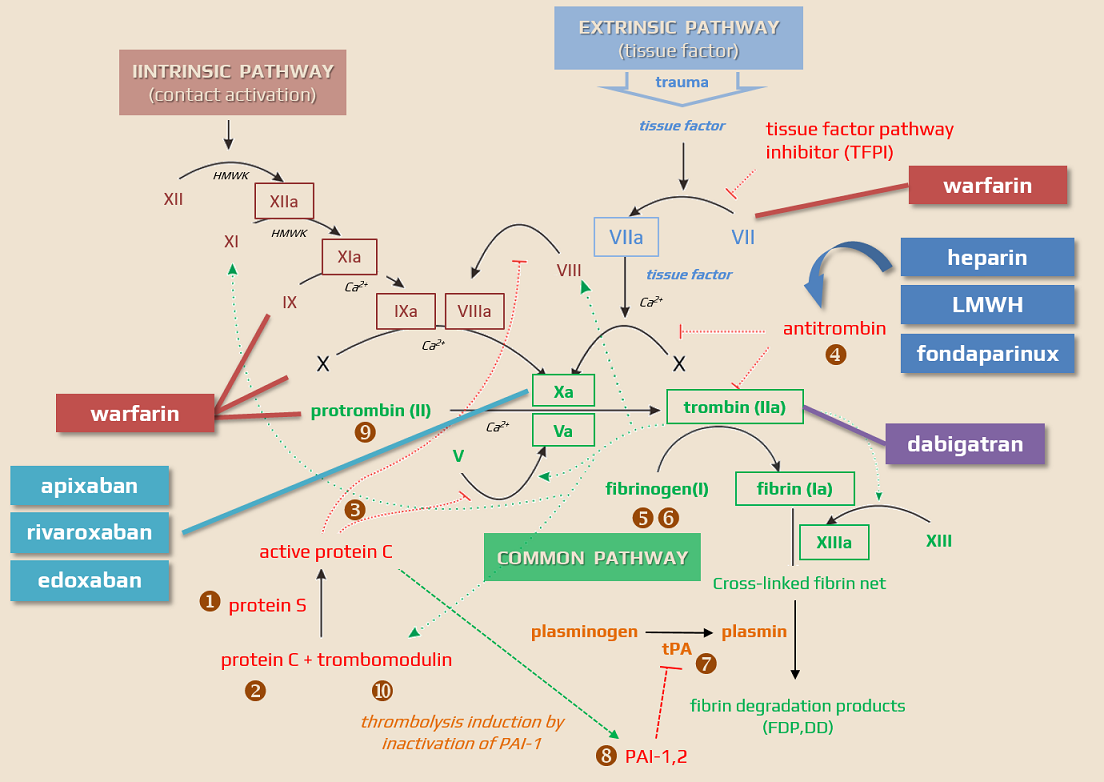
Mechanism of action
- Direct Thrombin Inhibitor (DTI) with a favorable safety profile without hepatotoxicity
- it reversibly binds to the active site on the thrombin molecule, preventing thrombin-mediated activation of coagulation factors
| Direct anticoagulants They inactivate the clotting factors present in the plasma |
Indirect anticoagulants They affect clotting factors by reducing their liver production |
|
| Direct thrombin/factor Xa inhibitors These drugs bind to thrombin/factor Xa and thereby block their function |
Indirect thrombin/factor Xa inhibitors These drugs activate antithrombin |
|
|
||
|
||
|
||
Pharmacokinetics and pharmacodynamics
- dabigatran is a prodrug – after absorption in the digestive tract, it undergoes enzymatic transformation into an active metabolite
- fasting or post-meal administration is possible
- rapid onset of action (peak at 2-3 hours after administration)
- half-life is ~ 8 hours after a single dose and increases to 14-17 hours after repeated administration
- the effect can extend up to 30 hours in cases of renal insufficiency!
- higher risk of bleeding with dTT > 200ng/mL detected 10-16h after the previous dose
- there is a relationship between dabigatran plasma concentration and its anticoagulant effect
- the kidneys excrete 80% of the drug ⇒ increased plasma concentrations in renal insufficiency
- prolongs APTT, but this effect is not dose- or effect-dependent (APTT is not equivalent to dabigatran serum concentration)
Interactions
| Content available only for logged-in subscribers (registration will be available soon) |
Dosing
- test kidney function (CrCl) before starting treatment
- CrCl < 30ml/min (0.5 mL/s) – dabigatran is contraindicated
- CrCl 30-50ml/min (0.5-0.83ml/s) – consider reducint the dose to 2×110 mg
- check CrCl every 3-6 months in patients with renal impairment → use the Cockcroft-Gault formula for calculation! (as per trials)
- reduce dose only if required; mild to moderate renal insufficiency is no reason for automatic dose reduction → reduced-dose DOACs [Eikelboom, 2011)
- educate the patient on the necessity of taking the pill every 12 hours (vague instructions about morning-evening administration are insufficient)
- if the patient forgets to take the medication, apply the 50% rule:
- < 6h have passed – take the medication
- > 6h have passed – skip this dose and continue with the next one as scheduled
| dabigatran (PRADAXA) | stroke prevention | VTE prevention and therapy (LMWH bridging for 5-10 days) |
VTE prevention in knee or hip surgery (THR 30 days, TKR 10 days) |
| CrCl > 0.83 ml/s (>50 ml/min) | 2x 150 mg | 2x 150 mg |
110 mg for the first day (starting within 4 hours after surgery) then 220 mg once daily |
| CrCl 0.5-0.83 ml/s (30-50 ml/min) |
2x 110 mg (or 2x 150 mg in the absence of other risk factors) |
2x 110 mg |
|
|
2x 110 mg | 2x 110 mg |
75 mg for the first day (starting within 4 hours after surgery) then 150 mg (2x75mg) once daily |
Monitoring
- clinical monitoring is standard for all anticoagulated patients (DOACs are no “shoot and forget” drugs)
- specific laboratory monitoring can be done in selected cases
Complications, adverse events
- in routine clinical practice, according to the GLORIA-AF registry, the annual risk of major bleeding is 1.12%, and life-threatening bleeding 0.54%
- dabigatran has fewer intracranial (IC) bleeds but more GI bleeds compared to warfarin (especially in patients >75 years of age [Desai, 2016]
- after resolving acute bleeding:
- exclude any source of bleeding (intestinal polyp, peptic ulcer, etc.), particularly with repeated bleeding
- exclude overdose – check renal functions, interactions, and other factors; check specific lab tests (peak and end-of-dose levels ) and adjust the dose if required
- consider a reduced dose or switch to another drug
|
Hemorrhagic complications (RE-LY)
|
|||
|
dabigatran 110mg
n=6015
|
dabigatran 150mg
n=6076
|
warfarin
n=6022
|
|
|
Major bleeding
|
322 (2.7%) / year
|
375 (3.11%) / year
|
397 (3.36%) / year
|
|
Intracranial bleeding
(ICH, SAH, SDH) |
27 (0.23%) / year
|
36 (0,3%) / year
|
87 (0,74%) / year
|
|
life-threatening bleeding
|
145 (1.22%) / year
|
175 (1.45%) / year
|
212 (1,8%) / year
|
| GI bleeding |
133 (1.12%) / year |
182 (1.51%) / year |
120 (1.01%) / year |
|
Other adverse events
|
Dabigatran
110 mg (%) |
Dabigatran
150 mg (%) |
Warfarin
|
|
dyspepsia
|
11,8
|
1,3
|
5,8
|
|
dyspnoea
|
9,3
|
9,5
|
9,7
|
|
dizziness
|
8,1
|
8,3
|
9,4
|
|
peripheral edema
|
7,9
|
7,9
|
7,8
|
|
fatigue
|
6,6
|
6,6
|
6,2
|
|
cough
|
5,7
|
5,7
|
6
|
|
back pain
|
5,2
|
6,2
|
5,9
|
|
joint pain
|
4,5
|
5,5
|
5,7
|
|
diarrhea
|
6,3
|
6,5
|
5,7
|
FAQs
- it reversibly binds to the active site on the thrombin molecule, preventing thrombin-mediated activation of coagulation factors
- routine monitoring of coagulation tests is not required; however, specific tests are available if required
- kidney function should be tested before starting treatment and periodically thereafter, especially in patients with renal impairment
- dabigatran does not have significant dietary restrictions like warfarin, but patients should maintain a consistent lifestyle and consult their doctor before making any major changes
- if a dose is missed and < 6 hours have passed, take it as soon as remembered
- if > 6 hours have passed, skip the missed dose and continue as usual
- do not double the dose
- dabigatran is generally not recommended during pregnancy. It’s important to consult with a healthcare provider for management of anticoagulation during pregnancy.
- yes, the effects of dabigatran can be reversed with a drug called idarucizumab, used in emergencies such as life-threatening or uncontrolled bleeding or urgent surgery
- dabigatran offers some advantages over warfarin, like a lower risk of certain types of bleeding and no need for routine blood tests to monitor its effectiveness
- it also has a more predictable anticoagulant effect and fewer dietary restrictions
- the choice between these medications depends on individual patient factors
- dabigatran needs to be used with caution in patients with renal impairment; the dosage might need adjustment based on kidney function, and it’s contraindicated in severe renal impairment