ADD-ONS
Regulation of cerebral blood flow
Updated on 05/11/2023, published on 16/05/2023
- the regulation of cerebral blood flow (CBF) is critical for ensuring that the brain receives a steady supply of nutrients and oxygen despite fluctuations in systemic blood pressure
- the brain, weighing ∼ 1400 g (∼2% of total body weight), has substantial metabolic demands:
- 20% of basal oxygen demand (~ 3.5 mL O2/100g brain tissue/min) (Rink, 2011)
- 25% of basal glucose demand (~ 75-100 mg/min; ~5.6 mg glucose/100 g brain tissue/min) (Mergenthaler, 2013)
- 14-20% of cardiac output (CBF is ~ 700-800 mL in total per minute for standard 1400g brain; 50ml/100g brain tissue/min)
- ∼ 70% in the anterior circulation
- ∼ 30% in the posterior circulation
- ∼ 70% in the anterior circulation
- the brain’s blood supply is provided by two internal carotid arteries (ICA) and two vertebral arteries, which anastomose intracranially to form the circle of Willis → Anatomy of cerebral arteries
- the intracranial vascular compartment has an arterial (high-pressure) segment with active autoregulation and a venous (low-pressure, relatively passive) segment; both can change their volume
- CBF decreases with age
Cerebral blood flow (CBF) definition
CBF = CPP / CVR
CPP = MAP – ICP (or CVP if higher than ICP)
MAP = CO x SVR
cerebral resistance (R) = (8 x l x η) / πr4
l = length of the vessel, η = viscosity of the blood, r = vessel radius
CPP – Cerebral Perfusion Pressure, MAP – Mean Arterial Pressure, CVR – Cerebral Vascular Resistance, SVR – Systemic Vascular Resistance, CO – Cardiac Output
- with a stable CPP, changes in cerebral blood flow (CBF) are regulated by cerebrovascular resistance (CVR), primarily through alterations in the diameter of intracranial arteries and arterioles
- narrowing of arterioles leads to an increase in CVR and a decrease in blood flow velocity in the proximal arterial segment
- CVR also rises with increased blood viscosity (polyglobulia, leukocytosis, increased plasma protein content, etc.)
- narrowing of arterioles leads to an increase in CVR and a decrease in blood flow velocity in the proximal arterial segment
- Cerebral Perfusion Pressure (CPP) is calculated as the difference between mean arterial pressure (MAP) and intracranial pressure or CVP (pathology)
- normal CPP ∼ 70-90 mm Hg (ICP = 10 mmHg under physiologic circumstances)
- CPP< 50 mm Hg leads to hypoperfusion (which becomes irreversible at CPP < 30mmHg)
- an increase in ICP (or CVP) leads to a decrease in CPP, assuming MAP remains stable
- normal CPP ∼ 70-90 mm Hg (ICP = 10 mmHg under physiologic circumstances)
- CBF is constant at MAP in the range of 60(70)-160 (170) mmHg (different values were reported); values outside this range cause passive changes in CBF (see autoregulation curve below)
- MAP < 50-70 mmHg (i.e. CPP < 50 mm Hg) leads to hypoperfusion
- MAP > 160-170 mmHg (i.e., CPP > 150 mm Hg) leads to hyperperfusion, impaired blood-brain barrier (BBB) integrity, and the development of edema or bleeding (e.g., post-CEA hyperperfusion syndrome)
- the precise MAP range for autoregulation is not clear
- normal CBF
- gray matter 70-80 mL/100g/min
- white matter 20-40 mL/100g/min
- pathologic CBF values:
- penumbra ∼12-20 mL/100g/min
- ischemia < 12 mL/100g/min
- in healthy individuals, CBF decreases by up to 28% during REM sleep but increases by up to 40% during non-REM sleep. These hemodynamic changes are accompanied by corresponding metabolic changes, variations in oxygen extraction from the blood, etc.
Regulation of cerebral circulation
Regulation maintains constant cerebral blood flow despite fluctuations in systemic blood pressure and, to a certain extent, local drops in perfusion pressure (such as distal to the stenosis)
Neurogenic regulation
- a complement to autoregulation and metabolic regulation is mediated by baroreceptors (nerve endings sensitive to changes in blood pressure)
- baroreceptors are located in the ascending aorta and carotid sinuses and are connected to the medullary cardiovascular center (which includes cardioaccelerator, cardioinhibitor, and vasomotor components)
- this system protects the brain against sudden elevations or drops in systemic blood pressure
- other neural mechanisms can also significantly impact cardiovascular function (such as the limbic system linking the physiological responses to psychological stimuli, etc.)
- baroreceptors are specialized receptors located in blood vessels and heart chambers, responding to stretch
- they send impulses to the cardiovascular center to regulate blood pressure
- vascular baroreceptors are located in the sinuses of the aorta and carotid arteries
- aortic sinuses – in the wall of the ascending aorta, just above the aortic valve
- carotid sinuses – at the origin of the internal carotid arteries
Increased blood pressure
- stretched baroreceptors initiate action potentials at a higher rate
- cardiac output decreases
- sympathetic stimulation of the peripheral arterioles decreases, resulting in systemic vasodilation (inhibition of the vasomotor center)
- these actions result in a reduction of blood pressure
Decreased blood pressure
- the degree of stretch diminishes, reducing the rate of baroreceptor firing
- this triggers increased sympathetic stimulation of the heart, thereby elevating cardiac output
- it also triggers sympathetic stimulation of the peripheral vessels, resulting in systemic vasoconstriction
- these activities contribute to an increase in blood pressure
| Content available only for logged-in subscribers (registration will be available soon) |
Metabolic regulation
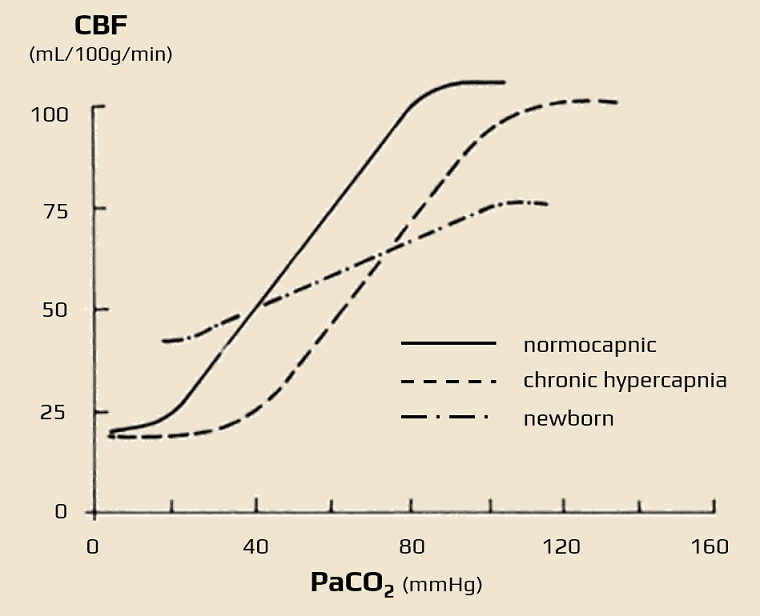
CBF changes in response to PaCO2 levels
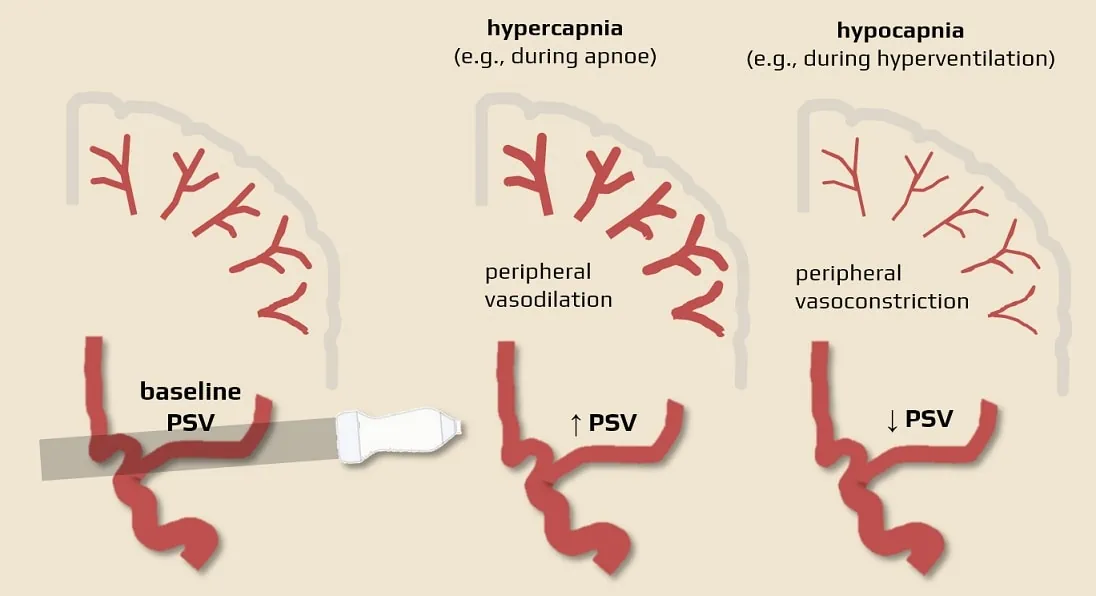
- ↓PaCO2 (hypocapnia, e.g., due to hyperventilation): CBF decreases (vasoconstriction → ↑peripheral resistance → decreased velocity in MCA, detectable by TCCD)
- a 1kPa decrease in pCO2 leads to ↓CBF by 15 mL/100g of brain tissue/min
- ↑PaCO2 (hypercapnia): CBF increases (vasodilation → ↓peripheral resistance → increased velocity in MCA, detectable by TCCD)
- 5% CO2 inhalation induces a 50% increase in CBF
- the increase in flow is by about 1-2mL/100g/min for every 1mmHg increase in CO2
- 5% CO2 inhalation induces a 50% increase in CBF
- hypercapnia alters the standard autoregulatory relationship between MAP and blood flow
CBF changes in response to reduced oxygen supply
- acute hypoxia (PaO2 <50 mmHg) is a potent dilator and exponentially increases CBF
- opening of KATP channels in smooth muscle ⇒ hyperpolarization and vasodilation
- hypoxia increases nitric oxide and adenosine production ⇒ vasodilation
Autoregulation
| Content available only for logged-in subscribers (registration will be available soon) |
- hypercapnia
- stroke
- traumatic brain injury (TBI)
- global hypoxic brain injury
- regionally, surrounding a space-occupying lesion or a hematoma
- infection (e.g., meningitis or encephalitis)
- malignant hypertension
- diabetic microangiopathy (after many years of uncontrolled diabetes)
- hepatic encephalopathy
- septic encephalopathy
Relationship between perfusion and regional metabolism
Overview of measurable parameters
- in general, locally increased cerebral metabolic activity (regional Cerebral Metabolic Rate – rCMR) leads to ↑ regional CBF (rCBF)
- AVDO2 – Arterio-Venous oxygen Difference
- CaO2-CvO2
- increases with rising brain activity or decreasing CBF
- OEF (Oxygen Extraction Fraction) or OER (Oxygen Extraction Ratio)
- (CaO2-CvO2)/CaO2
- when CBF decreases due to ischemia, OEF increases
- when CBF decreases due to reduced metabolism, OEF remains unchanged
- CMRO2 (Cerebral Metabolic Rate of Oxygen consumption)
- CMRO2 = (AVDO2 x CBF) (mL/100g/min) = CBF x OEF x CaO2
- decrease in CBF due to occlusion is transiently compensated by increased oxygen extraction from the blood
- rCBF, OEF, and CMRO2 can be measured with PET and MRI [Lin, 2017]
Consequences of a decrease in perfusion pressure
- initially, peripheral vasodilation occurs due to autoregulation (ensuring stable CBF) with a simultaneous increase in CBV (stage I)
- a drop in local perfusion pressure below the lower limit of autoregulation leads to a reduction in regional CBF (rCBF)
- this leads to a compensatory increase in oxygen extraction from the blood “oxygen extraction reserve”) ⇒ increased AVDO2 (stage II)
- a state of reduced cerebral flow with preserved metabolism (oligemia) is thus established
- with a further decline in perfusion pressure (or CBF) despite maximal O2 extraction, oxygen supply diminishes (stage III)
- reversible failure (penumbra) occurs when flow decreases to ~ 12-25mL/100g brain tissue/min
- irreversible damage (infarct, core) occurs with a decrease to < 12 mL/100g/min

![Right MCA occlusion with internal border zone infarct on MRI. PET shows decreased CBF and CMRO2 and increased OEF [Yamauchi, Stroke 2009] Right MCA occlusion with internal border zone infarct on MRI. PET shows decreased CBF and CMRO2 and increased OEF [Yamauchi, Stroke 2009]](https://www.stroke-manual.com/wp-content/uploads/2023/05/CBF-PET2.webp)

