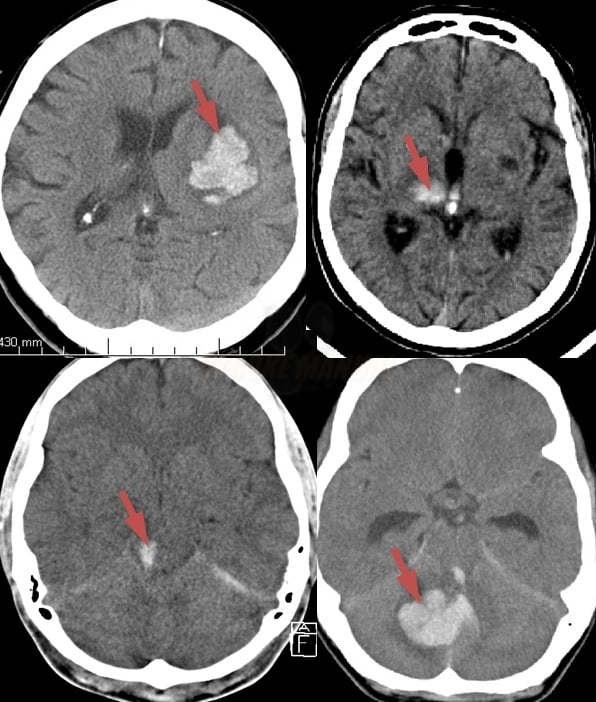
INTRACEREBRAL HEMORRHAGE
Etiology and clinical presentation of intracerebral hemorrhage
Updated on 21/03/2024, published on 16/04/2021
- intracerebral hemorrhage (ICH) is characterized by spontaneous rupture of blood vessels within the cerebral parenchyma, leading to focal hematoma formation and subsequent mass effect
- ICH accounts for approx. 10-20% of all strokes
- the 30-day mortality is up to 40%, the annual mortality is ~50-60%, and severe deficits are common in survivors [Broderick, 1993]
- bleeding during antithrombotic therapy is associated with increased mortality (including DOACs and antiplatelets) – HR 1.3 on antiplatelet therapy, 1.4 on anticoagulation [Apostolaki-Hansson]
- ICH is a heterogeneous group in terms of etiology, clinical presentation, and therapy
- a hematoma in the posterior fossa is always an acute, life-threatening condition
- limited compliance can quickly lead to herniation upwards, transtentorially, or downward through the foramen magnum
Intracranial vs. intracerebral hemorrhage
Classification
| Classification according to the etiology |
|
| Classification according to ICH location |
Supratentorial hemorrhage (85%)
|
Infratentorial hemorrhage (15%)
|
|
Intraventricular hemorrhage (primary, secondary)
|
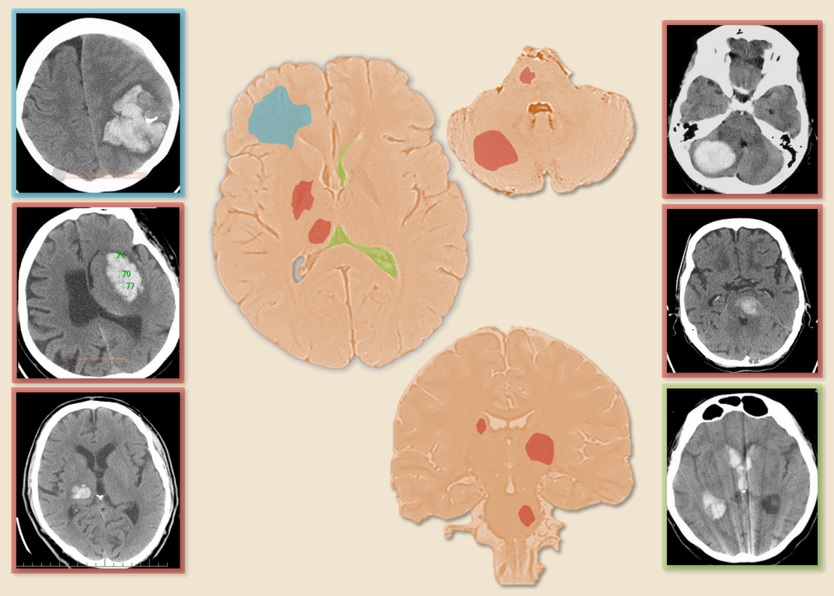
Etiology
- hypertensive arteriolopathy is the most common cause of intracerebral hemorrhage (ICH)
- the relative risk of ICH in a patient with arterial hypertension compared to a person without hypertension is approximately 4
- hypertension leads to bleeding by two mechanisms:
- rupture of an artery affected by chronic hypertension
- acute or subacute severe hypertension leading to rupture of a previously unaffected artery (malignant hypertension)
- typical localization: basal ganglia, thalamus, cerebellum, pons
- a secondary extension of the hematoma into the ventricles (hemocephalus) or subarachnoid space is possible
- a secondary extension of the hematoma into the ventricles (hemocephalus) or subarachnoid space is possible
- hypertension leads to hypertrophy and degeneration of the media of small arteries (lipohyalinosis, fibrinoid necrosis)
- the findings that suggest hypertensive etiology:
- history of hypertension
- typical ICH localization
- absence of any other apparent cause of bleeding
- left ventricular hypertrophy
- leukoaraiosis on CT/MRI
- hypertensive retinopathy
- high blood pressure on admission is not a conclusive indicator of hypertensive disease; it may be a result of stress reaction and intracranial hypertension
- tumors [Chrastina, 2011]
- venous infarction
- ischemia with hemorrhagic transformation, atypical location for ischemic stroke (typically on convexity), and edema
- hemorrhagic arterial infarction
- spontaneous or post-thrombolysis late reperfusion → classification of hemorrhagic transformations after thrombolysis
- mycotic aneurysms
- granuloma
- abscess
- aneurysm
- 20-40% of SAHs have an IC hematoma component
- rarely, rupture manifests as isolated ICH [Li, 2016]
- 20-40% of SAHs have an IC hematoma component
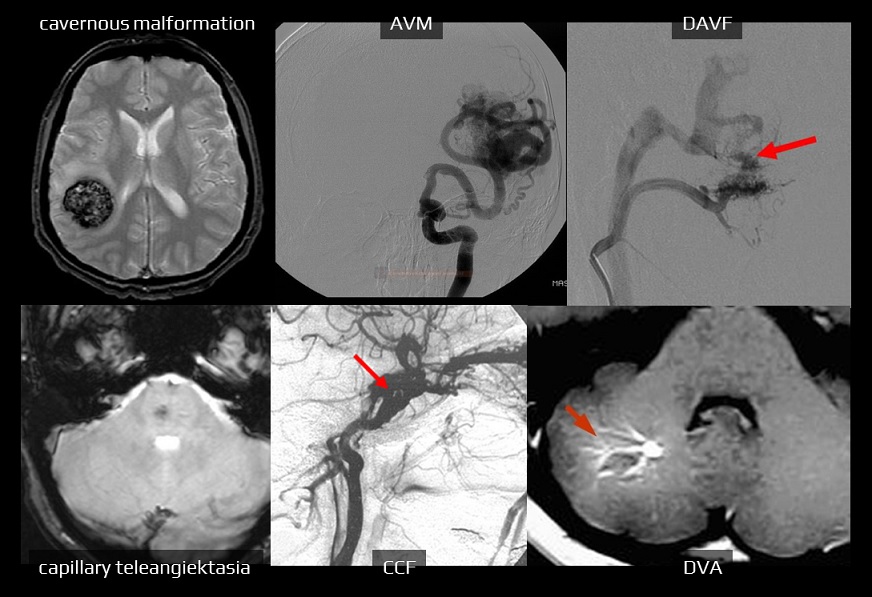
- vascular malformations
| MRI | 0.25-0.7% rebleeding 4.5% |
|
| MRI, DSA | 0.2-0.4% | |
| DSA, CTA | 2-4 % rebleeding 6-18% |
|
| MRI | very low |
|
| DSA, MRA, CTA |
type I – very low
type II, III – up to 8%
|
|
| Carotido-cavernous fistula (CCF) | DSA, MRA | very low |
- anticoagulants (LMWHs, UFH, warfarin, DOACs)
- the risk of ICH with long-term anticoagulant therapy is ~2% (risk is lower with DOACs)
- risk of major bleeding with warfarin is 1.7% in those aged < 75 years, ~4.2% in those aged > 75 years; the risk of ICH is 0.6 vs. 1.8 (according to SPAF II)
- hematomas are often non-homogeneous and multilobar (with a positive black hole and blend sign)
- antiplatelet therapy
- bleeding is more common with dual antiplatelet therapy (DAPT) and in combination with other risk factors
- fibrinolytics
- risk of symptomatic ICH (sICH) in acute stroke treated with tPA is ~ 6%
- other coagulation disorders
- leukemia, liver disease (related to alcoholism)
- thrombocytopenia or thrombocytopathy
- cerebral amyloid angiopathy (CAA)
- age >60 years or a positive family history
- cognitive deterioration
- β-amyloid deposits in small and medium-sized cerebral arteries
- lobar hematomas, repeated/multiple hemorrhages (including microscopic ones)
- leukoaraiosis on MRI and/or microbleeds on GRE
- risk of recurrence ~10%/year (higher in individuals with APOE E2 and E4 positivity)
- intracranial artery dissection (which is more likely to cause SAH or a combination of SAH+ICH)
- vasculitis (polyarteritis nodosa, Wegener’s granulomatosis, SLE, Henoch-Schönlein, syphilis, primary CNS granulomatosis, etc.)
- usually, hemorrhagic transformation of ischemia that must be distinguished from a primary hematoma
- occasionally, it may be difficult to distinguish between a traumatic hematoma and a spontaneous hematoma that was the cause of the fall
- signs of a traumatic etiology:
- cocaine, pseudoephedrine, amphetamine
- drugs often cause lobar hematomas
| Etiologic Classification of Intracerebral Hemorrhage – SMASH-U [Meretoja, 2012] | ||
| incidence | mortality at 3 months |
|
| Structural lesions (cavernous malformation, AVM) | 5% | 4 % |
| Medication (warfarin, DOAC, antiplatelet therapy) | 14% | 54 % |
| Cerebral Amyloid Angiopathy (CAA) |
20% | 22 % |
| Systemic disease (liver, kidney disease, thrombocytopenia/thrombocytopathies) | 5% | 44 % |
| Hypertension | 35% | 33 % |
| Undetermined | 21% | 30% |
Clinical presentation
- sudden, apoplectic onset
- personal medical history
- dementia? (potential amyloid angiopathy)
- hematologic disorder, antiplatelet, or anticoagulant therapy?
- history of hypertension?
- alcoholism, hepatopathy, renal disease?
- history of bleeding or known malformations?
- recent CEA or CAS? (risk of hyperperfusion injury)
- focal neurologic symptoms (such as hemiparesis, aphasia, hemianopsia, etc., depending on hematoma location) → Signs and symptoms of cerebral lesions
- altered level of consciousness (up to 50%) – more common in ICH compared to ischemic stroke
- the patient is usually somnolent or even soporous
- initial coma occurs with extensive thalamic or brainstem hemorrhage, destructing the reticular formation
- hypertension or hypertensive crisis (in up to 90%)
- acutely decompensated chronic hypertension
- stress-induced hypertension in otherwise normotensive patients
- acutely decompensated chronic hypertension
- nausea and/or vomiting (24-50%)
- headache (40%)
- epileptic seizures (about 6%)
- early improvement or fluctuation is not typical for ICH
Complications
Bleeding progression
- about 1/3 of patients with ICH experience a 1/3 increase in hematoma within 3 hours of onset (2/3 of them within 1 hour)
[Kazui,1996]
- perform a follow-up CT scan within 24 hours or immediately if the neurological status worsens
- some protocols suggest performing a CT scan every 12 hours until the hematoma volume has stabilized
- progression is most common in hematologic disorders but can also occur in typical hypertensive bleeding
- progression of bleeding is associated with early neurological deterioration and poorer prognosis
- radiologic predictors of progression:
- noncontrast CT scan – Blend sign, Black hole sign
- CT angiography – Spot sign
- hematoma progression in the following days is less typical and may indicate recurrent bleeding
- particularly with hematologic disorders or vascular malformations (most commonly in aneurysms or AVMs)
Brain edema and intracranial hypertension
- edema and intracranial hypertension develop shortly after the onset of bleeding, peaking between days 2 and 6
- bleeding in the posterior fossa may lead to acute obstructive hydrocephalus
Obstructive (non-communicating) hydrocephalus
- the highest risk is associated with:
- extensive intraventricular hemorrhage (primary or secondary)
- extensive cerebellar or brainstem hematomas causing direct compression of the cerebral aqueduct
- extensive intraventricular hemorrhage (primary or secondary)
- ⇒ indication for acute surgery (EDV)
Epileptic seizures
- early-onset sezures are most commonly associated with parenchymal lesions → stroke-related epilepsy
Extracranial (systemic) complications
- extracranial complications are similar to those observed in ischemic stroke → see here
- some notes regarding blood pressure:
- elevated blood pressure (BP) may result from decompensated chronic hypertension or be a stress reaction in previously normotensive individuals
- elevated BP upon admission does not automatically imply a hypertensive etiology of the bleeding
- normal BP upon admission increases the likelihood of bleeding from a vascular source ⇒ perform vascular imaging (CTA, MRA, or DSA)
Prognosis
- always make an initial rough estimation of the prognosis
- 30-day mortality 35-50%, up to 70% for recurrent ICH
- prognosis depends on the following:
- age and general biological status (incl. comorbidities)
- initial level of consciousness (LOC)
- ICH location
- hematoma size (ICH score)
- GCS < 9 and ICH volume > 60ml ~ 90% mortality
- GCS ≥ 9 and ICH volume < 30ml ~ 17% mortality
- poor outcome is associated with an ICH score of 4-6
- presence of spot sign, blend sign, and black hole sign
- etiology of bleeding (SMASH-U)
- acute phase complications (sepsis, ischemic stroke, prolonged mechanical ventilation, etc.)
- functional recovery after ICH is highest in the first few weeks to months (greatest within 30 days)
- early and long-term rehabilitation and ergotherapy are essential
- for assessing the functional outcome, the Modified Rankin Scale (mRS) is frequently used
- assess cognitive functions, as impairment is frequently observed among patients after ICH
Risk of recurrence
- risk of ICH recurrence depends on etiology and risk factors
- the estimated recurrence risk is 1.2-7% per year across undifferentiated patients with ICH (with the highest event rate occurring in the first year after bleeding)
- risk factors for the recurrence of ICH
- advanced age
- race (Black, Asian)
- poorly controlled hypertension
- history of prior ICH and ischemic stroke
- ICH location (nonlobar<lobar)
- etiology (increased risk with coagulation disorders, CCA, brainstem cavernous malformations, Moyamoya disease, AVM, tumors)
- imaging features (lobar microbleeds, leukoencephalopathy, cortical superficial siderosis)
- CHKD (can be a marker of atherosclerotic disease)
- genetic features (carriers of apolipoprotein-E e2 or e4 genotypes)
| ICH recurrence risk (annual) | |
| Hypertonic bleeding (precise BP correction reduces RR by 50%) | 1.1-4 % |
| Cerebral amyloid angiopathy (CAA) |
7.5-20 % |
| AV malformation (AVM) |
6-18 % |
| Cerebral cavernous malformation (CCM) |
3.8-30% |
| Dural AV fistula | 0.15 % |
Follow up imaging
- patients who fail to improve or deteriorate during the recovery phase need imaging to rule out recurrent bleeding
- stabilized patients with hypertensive ICH don´t require additional imaging
- features indicative of hypertensive bleeding: history of hypertension (HTN), subcortical microbleeds, no atypical imaging features, age ≥65 years
- no definitively established etiology or baseline imaging features suggestive of an underlying cause require follow-up imaging performed after bleeding and edema have resolved
- perform brain MRI 4-16 weeks after the ICH (incl. GRE/SWI and contrast-enhanced images); if an underlying vascular cause is suspected, add noninvasive vascular imaging, such as CTA or MRA
- MRI is optimal for detecting cerebral venous sinus thrombosis, vascular malformations, hemorrhagic transformation of an ischemic infarct, or neoplasms
- contrast-enhanced CT of the brain is a reasonable alternative for those who are unable to undergo an MRI
- stabilized CAA patients may not require additional imaging
Clinical features raising suspicion for an underlying cause
- age <65 years
- no history or new diagnosis of HTN
- history of new-onset headaches
- history of new-onset neurologic symptoms preceding ICH
- thunderclap headache at the onset of hemorrhage
- history of prior ICH (unless attributed to uncontrolled HTN or CAA)
Imaging features on baseline imaging raising suspicion for secondary cause of ICH
- early perihematomal edema disproportional to the size of the hematoma
- nonconfluent hemorrhage in the arterial vascular territory (probable ischemic infarction)
- enhancement of intracranial vessels around ICH
- multifocal hemorrhage
- isolated intraventricular hemorrhage
ICH prevention
- long-term precise blood pressure (BP) control is required in all ICH patients (AHA/ASA 2022 1/B-R)
- this approach is supported by evidence from ischemic stroke prevention trials (SPS3, RESPECT)
- this approach is supported by evidence from ischemic stroke prevention trials (SPS3, RESPECT)
- start therapy ASAP, combining pharmacological and nonpharmacological approaches
- the first-choice drug is usually an ACE inhibitor; if not tolerated, use an angiotensin receptor blocker, thiazide diuretic, or calcium channel blocker
- usually, a combination of drugs is necessary
- acute BP management is discussed elsewhere (target 140-160 mmHg)
- long-term BP should be maintained < 130/80 mmHg (AHA/ASA 2022 2a/B-NR)
- aim for a BP < 120/80 mmHg in younger patients without major comorbidities (Teo, 2022)
- a stepwise correction to achieve target values is suggested in the subacute phase (within 2 weeks)
- outpatient monitoring is essential to ensure long-term proper BP control
- reduced salt intake, healthy diet
- cessation of smoking and excessive alcohol intake
- treatment of sleep apnea, if present
- regular physical activity
- maintenance of a healthy body weight
- avoidance of sympathomimetics
| Content available only for logged-in subscribers (registration will be available soon) |
- epidemiological studies and clinical trials provide conflicting data
- in patients with spontaneous ICH and an established indication for statin pharmacotherapy, the risks and benefits of statin therapy are uncertain (AHA/ASA guidelines 2022
- the decision to use statins in patients with ICH depends on the individual assessment of the risk of ischemic events versus recurrent ICH
- there is no strong evidence to discontinue the hypolipidemic therapy after ICH
- a large 10-year nationwide cohort study from Taiwan found no association between statin dose and risk of recurrent ICH (Tai, 201)
- statins are associated with improved functional outcome and reduced mortality in patients with prior ICH (Ziff, 2018)
- data from the Danish registry show, that exposure to statins is not associated with an increased risk of recurrent ICH but was associated with a lower risk of any stroke (Gaist, 2023)
- in patients with a high risk of hemorrhage (typically after recurrent parenchymal hematoma), a more cautious approach may be warranted, potentially involving alternative lipid-lowering drugs
- in patients with spontaneous ICH, regular long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) is potentially harmful because of the increased risk of ICH (AHA/ASA 2022 3/B-NR)
- prefer nonacetylated salicylates
- the presence and extent of cerebral microbleeds and cortical superficial siderosis predict subsequent symptomatic ICH
- incorporate available MRI results into decision-making regarding stroke prevention plans (avoid warfarin, apply strict BP management, etc.) (AHA/ASA 2022 2b/C-LD)