ISCHEMIC STROKE / ACUTE THERAPY
Recanalization therapy in acute stroke
Updated on 24/07/2024, published on 16/09/2022
Introduction
- acute stroke therapy aims to restore blood flow, allowing oxygen and nutrients to reach the ischemic brain tissue
- recanalization refers to the reopening of an occluded artery
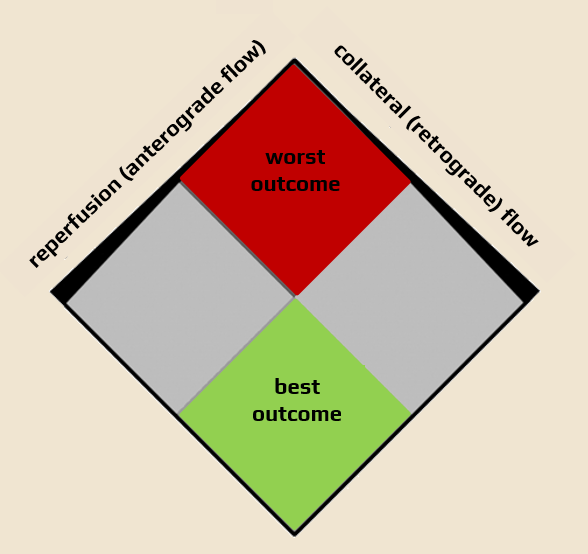
- reperfusion refers to the restoration of oxygen and nutrient supply to the ischemic brain tissue; this can happen via collaterals (retrograde perfusion, which is usually inadequate) or due to recanalization (anterograde reperfusion)
- to achieve good clinical outcome, not only recanalization but also high-quality and timely reperfusion is required
- time is of the essence, as reperfusion must occur before the penumbra turns into necrosis
- recanalization techniques include thrombolysis and mechanical thrombectomy or their combination
|
Recanalization
|
restoration of flow at the primary occlusion site | AOL |
| Reperfusion | restoration of anterograde flow in the vessels beyond the primary occlusion site (anterograde perfusion) | TICI |
| Collateral circulation |
retrograde perfusion | |
| Distal embolization | new occlusion detected distal to the primary occlusion site | |
| Reocclusion | occlusion of an already opened artery |
Time is brain
- most ischemic strokes are caused by occlusion of a cerebral artery (up to 80% according to angiographic studies); this occlusion occurs due to thrombosis or embolization, leading to subsequent hypoperfusion of brain tissue.
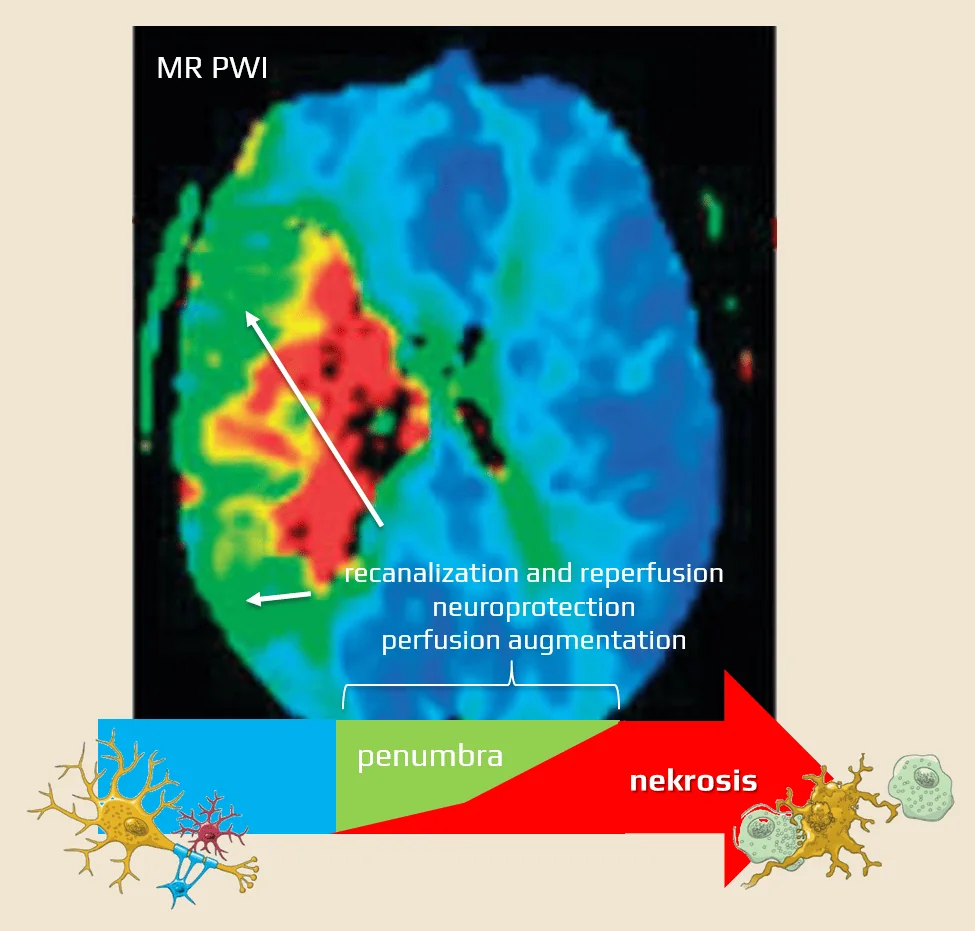
- when blood flow decreases from normal values > 50 mL/100g/min to a range of 12-18 mL/100g/min, a reversible functional impairment (penumbra) occurs
- the penumbra is a region of brain tissue that is still viable but is at risk of irreversible damage if blood flow is not restored promptly
- if perfusion drops to <10 mL/100g brain tissue/min, irreversible damage to brain tissue occurs (a region called the necrotic core)
- ~ 2 million brain cells are lost per minute during this phase
- ~ 2 million brain cells are lost per minute during this phase
- penumbral neurons can remain viable for minutes to hours after arterial occlusion (representing an individual therapeutic window) and early restoration of blood flow to the ischemic brain tissue can reverse the damage and improve the outcome
- the primary goal of acute stroke treatment is to restore blood flow to the penumbra as quickly as possible
- this is typically achieved through recanalization of the occluded artery; early recanalization (and reperfusion) significantly improves the outcome of stroke patients
- in summary, the best outcome is achieved in patients with good collateral circulation and a large ischemic penumbra in whom rapid recanalization is achieved
Prognostic factors of a good outcome
The chances for recovery depend on many controllable and uncontrollable prognostic factors
- early recanalization and reperfusion are crucial for achieving a favorable outcome
- there is a strong correlation between recanalization and a favorable outcome (with an odds ratio of 4.4 favoring good outcome in recanalized patients)
[Rha, 2007]
- patients who recanalize are up to 13 times more likely to regain their independence at the 3 months [Smith,2004]
- among various recanalization techniques, mechanical thrombectomy (MT) has the highest recanalization potential
- however, recanalization does not always guarantee clinical improvement due to:
- distal embolization (16%) [Janjua, 2008]
- reocclusion (18%)
- no-reflow phenomenon (microcirculation blockage without adequate reperfusion despite recanalization) (Schiphorst, 2021)
- late reperfusion with BBB disruption, resulting in edema, hemorrhagic transformation
- the presence of viable hypoperfused tissue (penumbra) is a prerequisite for successful treatment
- detection of persistent penumbra (by CTP, PWI/DWI mismatch, or DWI/FLAIR mismatch) has enabled the treatment of patients beyond standard therapeutic windows
- relevant trials:
- another milestone was achieved by trials demonstrating the efficacy of thrombectomy within 24 hours, even in patients with large cerebral infarcts – these trials significantly changed clinical practice
- SELECT2 (2023) – ASPECT 3-5 or core ≥ 50 mL
- ANGEL-ASPECT (2023) – ASPECT 3-5 or core 70-100 mL
- RESCUE-JAPAN LIMIT trial (2022) – ASPECT 3-5
- advanced age is generally associated with a higher risk of stroke and a worse prognosis
- patients without occlusion generally have a better prognosis than those with proven arterial occlusion (OR 5.0)
- Clot Burden Score (CBS) <10 is associated with a lower chance of favorable outcome compared to a CBS of 10
- the odds ratio (OR) is 0.09 for CBS ≤ 5, 0.22 for CBS 6-7, and 0.48 for CBS 8-9 [Puetz, 2008]
- patients without proven arterial occlusion tend to have a lower baseline NIHSS (median 18 vs. 7) [Derex, 2002]
- the longer the thrombus, the lower the chance of achieving recanalization, with IVT having a minimal chance of recanalization for thrombi > 8mm
[Riedel, 2011]
- NIHSS correlates with the likelihood of arterial occlusion and is a predictor of outcome
- NIHSS ≥ 10 ⇒ high probability of large vessel occlusion (LVO) [Nakajima, 2004]
- good collateral circulation correlates with a smaller infarct core and predicts better clinical outcome with reperfusion therapy [Lima, 2010] [Kucinski, 2003] [Bang, 2011]
- mechanical recanalization trials show that the quality of collaterals determines the clinical outcome and could be used to select patients for endovascular therapy [Bijoy, 2015]
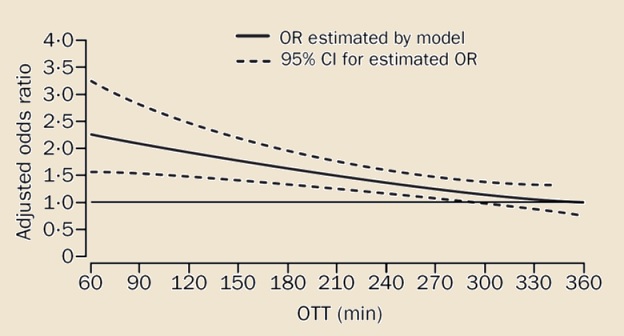
- the interval from stroke onset to treatment initiation (OTT) has a significant impact on outcome
- administering tPA earlier offers greater benefits, especially when started within 90 minutes
- the chance for a favorable outcome is twice as high if treatment is started within 90 minutes (OR 2.8) compared to treatment started after 181 minutes (OR 1.4); hence the slogan “time is brain” [Hacke, 2004]
- data from the IMS 3 trial suggest that each 30-minute delay reduces the chance of a favorable outcome by 10%
- simultaneously, initiating thrombolysis earlier also increases the chances of recanalization, as the composition of the thrombus and its ‘resistance’ to tPA change in the first few hours
- therefore, it is necessary to reduce transport times and Door-To-Needle Time (DTN time)
- the same principles apply to mechanical thrombectomy → start ASAP!
- extensive early ischemic changes indicate a severe stroke
- ASPECT score correlates with the baseline NIHSS value and can predict both the outcome and the risk of symptomatic hemorrhage
- a score of ≤ 6 is associated with a worse outcome
- conversely, a score of ≥ 7 predicts a favorable response to reperfusion therapy (up to a 3-fold chance of achieving independence compared with a score of ≤ 6) [Hill, 2003]
- a score of ≤ 6 is associated with a worse outcome
- recent trials have shown the benefit of MT even in patients with low ASPECTS (SELECT2, ANGEL-ASPECT)
Overview of recanalization methods
Intravenous thrombolysis → see here
- < 4.5 h – unselected eligible patients
- 4.5-9h / wake-up stroke/ unknown onset time – patients selected by advanced imaging detecting viable tissue
- < 24 hours – basilar artery occlusion (without advanced imaging)
Mechanical recanalization → see here
Combined procedures
- bridging IVT + thrombectomy
- thrombectomy + intra-arterial thrombolysis
- thrombectomy + carotid angioplasty (CAS)
Intravenous thrombolysis (IVT)
|
Unselected patients ≤ 4.5 hours after stroke onset
- or should be administered ASAP, even if mechanical thrombectomy is planned
- TCD/TCCD monitoring increases the likelihood of recanalization during thrombolysis, but the clinical benefit has not been demonstrated in trials ⇒ sonothrombolysis is not currently recommended beyond clinical trials
→ Intravenous thrombolysis
→ Fibrinolysis and fibrinolytic drugs
Unselected patients > 4.5 h after stroke onset
- except for basilar artery occlusion (BAO), the effect of IVT beyond 4.5 hours in unselected patients has not been demonstrated in RCTs
- advanced imaging beyond the 4.5-hour window is recommended (ESO guidelines 2021)
- however, it appears safe to treat patients with wake-up strokes and normal NCCT; similar results have been reported in patients with witnessed-onset and wake-up strokes with normal CT (Armon, 2019)
- negative results have been published with desmoteplase in a 3-9 hour time window without perfusion selection – DIAS 3 and DIAS 4
Patients selected by multimodal imaging (4.5-9 hours after stroke onset/WUS/uncertain time of onset)
- advanced imaging modalities are used in patient selection (CT/MR perfusion, MR DWI/FLAIR mismatch) – see below
- according to the meta-analysis of the WAKE-UP, EXTEND, THAWS, and ECASS-4 trials, IVT is considered safe and effective in patients selected by advanced imaging [Thomalla, 2020]
Endovascular treatment (EVT)
|
Mechanical thrombectomy (MT)
- MT is performed either after prior IVT or as primary therapy (direct MT) when thrombolysis is contraindicated
- for large vessel occlusions, combination therapy is undoubtedly superior to medical therapy alone
- similar to IVT, the time to recanalization (OTR) is critical in mechanical thrombectomy
- shorter OTR (Onset To Recanalization) time is associated with better MRs/3m (1.4 vs. 2.4 vs. 3.3, for OTR 124-240 vs. 241-360 vs. 361-660 minutes; p < 0.001) [Sheth, 2015]
- without advanced imaging selection, the therapy can be started < 6 hours after stroke
- in selected patients, treatment can be started within 24 hours
- the DAWN trial (2017) showed an effect up to 24h in strictly selected patients (based on CTP)
- in 1/2018, positive results of DEFUSE 3 showed efficacy within a 16-hour time window (OR 2.77!!!)
- recent trials SELECT2 (2023), ANGEL-ASPECT (2023), and RESCUE-JAPAN LIMIT trial (2022) have demonstrated the efficacy of MT even in patients with large infarcts
- the primary goal is rapid and complete recanalization of the occluded vessel
- retrievers are preferred; the same effect can be achieved with the Penumbra aspiration device (ASTER and COMPASS trials)
- not only the choice of the device is important, but also the way it is used – e.g., the ADAPT (A Direct Aspiration First Pass Technique) procedure with the PENUMBRA device [Turk, 2013]
- first-pass recanalization is also the goal of the 4th generation retrievers (Solitaire Platinum, EmboTrap)
- carotid artery stenosis/occlusion can be treated with acute angioplasty in case of tandem lesions (intracranial and extracranial)
- most authors recommend passing through the occluded/stenotic carotid artery distally to open the occluded intracranial artery first and then treat the extracranial pathology with angioplasty or stenting
Intra-arterial thrombolysis (IAT)
- the therapeutic window for IAT is < 6h from the onset of stroke symptoms
- IAT has been largely abandoned and replaced by thrombectomy
- can still be used as a supplement method, e.g., to manage distal embolization
Emergent carotid endarterectomy (CEA)
|
- may be considered for isolated symptomatic acute occlusion or significant ICA stenosis without concomitant intracranial artery occlusion (if the hemodynamic cause of the current deficit is assumed or proven by, e.g., TCCD) → see here
The purpose of multimodal imaging in patient selection
- advanced imaging should not delay the administration of IVT!
- tPA bolus should be administered immediately after completion of NCCT, followed by CTA/CTP
- CTA/CTP may sometimes help differentiate stroke mimics
Indications for IVT in strokes with unknown time of onset and within 4.5-9h window
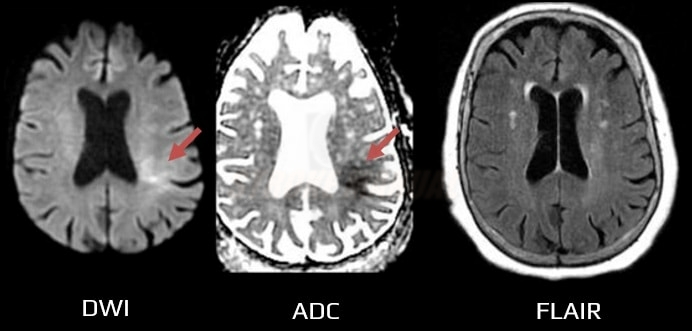
- a positive DWI with negative findings on FLAIR indicates that the stroke probably occurred within the previous 4.5 hours [Aoki, 2010] [Thomalla, 2009]
- may be useful to guide intravenous thrombolysis (IVT) in patients with stroke of unknown onset or wake-up stroke (WUS)
-
- DWI/FLAIR mismatch is present (DWI positive in <1/3 of the MCA territory, FLAIR still negative)
- n=503 ( 254 tPA vs. 249 placebo), median NIHSS 6
- good outcome 53.3 vs. 41.8 (placebo), median mRS/3m 1 vs. 2
- mortality 4.1% vs. 1.2% (placebo)
- sICH 2% vs. 0.4% (placebo)
- MR WITNESS
- DWI/FLAIR mismatch (DWI positive in <1/3 of the MCA territory, FLAIR either negative or showing only minimal lesion)
- IVT administered within 4.5 hours of symptom onset
- n = 80, sICH 1.25% (as defined by ECASS III)
- ≥ 18 years, premorbid mRS < 2, NIHSS 4-26
- median NIHSS was 12 (tPA) and 10 (placebo)
- CTP system RAPID or MR DWI/PWI mismatch → inclusion criteria
- mismatch > 1.2, absolute difference > 10 ml (Tmax > 6 s on perfusion)
- core < 70 ml (CT-CBF) – rCBF<30% on CTP or ADC<620mm2/s (DWI
- n=113 (tPA) vs. 112 (placebo) at 4.5-9h
- primary outcome (mRS 0-1 /3m): 35.4% vs 29.5%, RR 44%
- NNT 17 – efficacy similar to IVT at 3-4.5h
- sICH 6% (tPA) vs 1% (placebo)
- mortality 12% (tPA) vs. 9% (placebo)
Indications for endovascular procedures > 6h after stroke onset
- multimodal CT imaging to detect penumbra was crucial for the success of interventional trials beyond 6 hours (DAWN, DEFUSE 3) → Mechanical recanalization
Evaluation of therapy results
- treatment efficacy is represented by:
- percentage of complete recanalization with complete distal reperfusion (TICI 3) → Angiographic grading of revascularization therapy
- incidence of bleeding (particularly symptomatic ICH)
- clinical outcome (mRS ≤ 2 is considered a favorable outcome)
- ODT (Onset to Door)
- DIT (Door to Imaging)
- DTN (Door to Needle)
- DPT (Door to Puncture)
- DRT (Door to Reperfusion)
- PRT (Puncture to Reperfusion)
- ORT (Onset to Reperfusion)
Thrombolytic drugs
- the most commonly used thrombolytic agent is
- alternatively, may be used (ESO Guidelines 2023) (AHA/ASA guidelines 2019 IIb-BR)
- both alteplase and tenecteplase are tissue plasminogen activators (tPA)
- historically, the term “tPA” has been used to refer to alteplase because it has long been the only tissue plasminogen activator in use
- nowadays, the name of the specific agent should be used
- the use of other thrombolytics for the treatment of stroke is not recommended in routine clinical practice

![Recanalised patients are up to 13 times more likely not to remain disabled after three months [Smith, 2004]. Recanalised patients are up to 13 times more likely not to remain disabled after three months [Smith, 2004].](https://www.stroke-manual.com/wp-content/uploads/2022/09/Acute-stroke-prognosis.jpg)