GENERAL THERAPY
General management of acute stroke patients
Updated on 22/06/2024, published on 20/06/2023
- the principles of management of patients with a suspected acute stroke are to:
- make an accurate diagnosis and initiate specific stroke treatment (recanalization, surgery, etc.)
- manage and prevent general medical problems
- general therapy is similar for all types of brain injury (ischemic or hemorrhagic stroke, TBI, etc.)
- cardiac and pulmonary care, fluid and ion balance restoration, metabolic maintenance, blood pressure control, etc.
- preventing and treating complications (infection, VTE, intracranial hypertension, bed sores, etc.)
- estimate the prognosis for survival and future handicaps, and discuss the prognosis with the patient and family
- evaluate stroke etiology and initiate individualized secondary prevention to prevent stroke recurrence
- start early rehabilitation (incl. speech therapy, ergotherapy, etc.)
- manage stroke consequences (spasticity, depression, etc.) and continue with longer-term rehabilitation and support
- proper management of general conditions and complications is equally important as the specific therapy, even though the latter may sometimes attract more attention
Stroke unit care
|
- in the acute stage of large artery occlusion (before recanalization), keep a zero-degree head-of-bed (HOB) position (ZODIAC trial)
- this position seems to enhance collateral circulation and improve prognosis after mechanical recanalization
- after initial evaluation and therapy, it is recommended to admit the patient to a stroke unit (AHA/ASA 2019 I/A)
- for a minimum of 24 hours, preferably for 48-72 hours
- care is provided by a specialized multidisciplinary team
- treatment in a stroke unit results in a relative reduction in mortality and dependency compared to treatment in a regular ward [Langhorne, 1997]
- treating 100 patients using this approach can result in a reduction of 5 patients who would die or remain dependent (NNT 20)
- the benefit is universal and applicable to all types of stroke and patients of varying degrees of severity
Basic monitoring |
- pulse oximetry
- use a finger or earlobe sensor
- oxygen should be provided to maintain oxygen saturation ≥ 95% (AHA/ASA 2019, I/C)
- do not administer oxygen in nonhypoxic patients (AHA/ASA 2019 III/B-R)
- blood pressure
- thrombolyzed patients are managed according to thrombolytic protocols ; otherwise, check BP every 30 minutes in stabilized patient
- manage hypertension using specific protocols (→ Blood pressure management in acute stroke, hypertensive urgency protocols, etc.)
- acute stroke patients usually have increased blood pressure due to preexisting hypertension, stress, activation of the sympathetic, ACTH–cortisol and renin-angiotensin-aldosterone systems, and the Cushing reflex)
- hypotension and hypovolemia should be promptly corrected to avoid cerebral hypoperfusion
- hypotension is uncommon in acute stroke and may occur due to various reasons (excessive fluid loss, sepsis, heart disease)
- treat the underlying cause, raise the foot of the bed, and replace fluids with crystalloid (saline) solutions
- low cardiac output may require inotropic support
- the usefulness of drug-induced hypertension in stroke patients is not well established (AHA/ASA 2019, IIb/B)
- ECG
- record 12-lead ECG on admission, then monitor with 3-lead ECG
- especially infarctions in the right insula can lead to autonomic system failure and cardiac complications (ST depression, T wave inversion, troponin elevation in the laboratory)
- look for arrhythmias (particularly atrial fibrillation)
- monitoring of the consciousness level and neurostatus (basic neurological examination, GCS, NIHSS)
- adjust the frequency of evaluations based on the patient’s condition and underlying diagnosis (more frequent in extensive SAH, ICH, malignant edema)
- in unconscious patients, repeatedly assess brainstem reflexes
- adjust the frequency of evaluations based on the patient’s condition and underlying diagnosis (more frequent in extensive SAH, ICH, malignant edema)
- search for additional symptoms such as headache, nausea, vertigo, singultus
Extended neuromonitoring
|
- recommended in patients with severe stroke (~ 24-48h); a 10-20 or 2-4 channel EEG system with adhesive electrodes is used
- monitoring of SE treatment (always) and depth of anesthesia
- NCSE diagnosis
- sensitivity and specificity of standard EEG is low
- clinical manifestations are none or only subtle and often escape attention (facial twitching, short eyeball deviation, autonomic signs)
- DDx of non-epileptic seizures
- monitoring of metabolic coma
- SSEP (prognostic value in coma vigile)
- BAEP (brain death diagnosis in patients with decompressive craniectomy)
- ERP (event-related potential)
- detection of P300 wave in a coma is associated with a good prognosis
- indications for ischemic stroke patients are ambiguous
- probably advantageous in patients with GCS ≤ 8 with extensive hematoma, SAH, or hydrocephalus
- intraparenchymal or intraventricular sensors are available
- doppler signs
- decrease in velocities, increase in resistance and pulsatility index (RI and PI), then disappearance of diastolic flows, and finally systolic spikes or biphasic flow curve → TCCD in the diagnosis of brain death
- ICP=(10.927* PI)-1.284
- ↓ vasomotor reactivity
- B-mode signs
- optic nerve sheath enlargement or head prominence (transorbital approach)
- midline shift (transtemporal approach)
- a special catheter is inserted into the bulb of the internal jugular vein to monitor venous blood O2 saturation
- global information is provided, and therefore may not reflect localized issues; take into account body temperature and the effect of medication)
- normal values: 55-80%
- SjO2 < 50% with normal arterial O2 saturation indicates either ↓ CBF (e.g., when cerebrovascular resistance is increased) or increased consumption (↑CMRO2)
- SjO2 > 85% indicates either hyperemia with ↑CBF or ↓CMRO2
- 2 sensors are placed on the forehead; emitted beams penetrate to about 4 cm
- from the reflected light, the oxygenation of the brain tissue is derived
- direct measurement of parenchymal oxygenation near the sensor
- standard 20-45 mm Hg
- ischemic threshold (depending on the type of sensor < 15-20 mm Hg)
- measurement of extracellular metabolite levels in the CNS ( glucose, lactate, pyruvate, lactate/pyruvate ratio, glutamate, glycerol)
Intravascular access |
Peripheral venous system
- ensure intravascular access via a venous cannula in every acute stroke patient (bilaterally if thrombolysis is planned)
- uncomplicated insertion and management
- prefer disinfection with 0.5% chlorhexidine in alcohol during insertion
- can not be used for certain drugs and concentrated solutions (< 600 mOsm/l), as higher osmolarities increase the risk of phlebitis and vein damage
- insertion time: 72-96 h
- remove the cannula if unused for > 24h or if no further use is probable
- replace the cannula in case of local (e.g., pain, swelling, skin discoloration, skin temperature change, hardening, resistance to flushing) or systemic complications (fever)
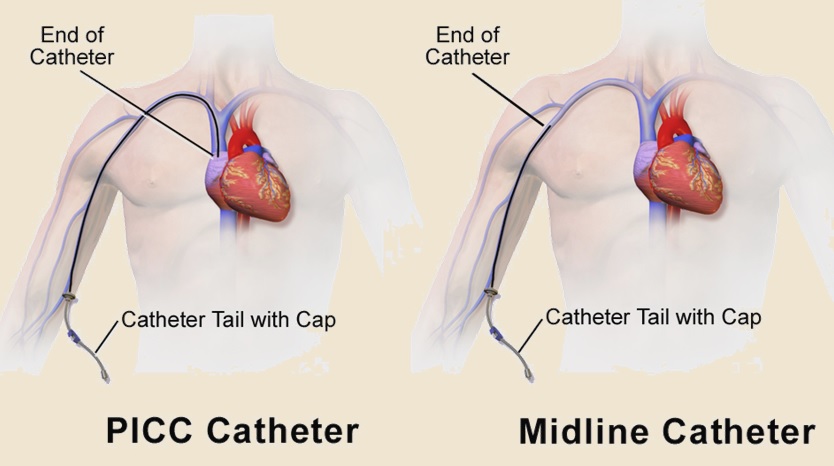
- for long-term use, prefer the midline catheter (can be used up to 2-4 weeks)
- can be used for blood sampling
- allows the administration of drugs with osmolarity < 600 mOsm/l and nutrition < 800 mOsm/l
- if peripheral IV access is required for > 3 months, a PICC catheter is preferable
- PICC is inserted through a peripheral vein, and the catheter’s tip is placed into the cavoatrial junction
- the technique typically involves observing the P-wave on the ECG. As the catheter’s tip approaches the lower third of the superior vena cava, the P-wave generally increases. When the tip reaches the cavoatrial junction, which is the optimal position, the P-wave is at its maximum amplitude
- it may be kept for several months
Central venous system
- central venous catheters (lines) are commonly placed in the internal jugular, subclavian, or femoral veins (typically usually using a two or three-way cannula)
- it is advisable to order a chest X-ray 3 hours after subclavian vein cannulation to exclude pneumothorax
- central catheter enables:
- administration of all types of drugs and concentrated solutions
- measurement of central venous pressure (CVP)
- the normal CVP range is 5-12 cm H2O; subtract positive end-expiratory pressure (PEEP) in ventilated patients
- adhere to aseptic treatment – use sterile cover and perform daily checks
- complications:
- the insertion time: 1-3 weeks
- risk of infection increases after day 7 [Öncü, 2003]
- use PICC or tunneled catheters when prolonged central venous access for prolonged central venous access
Major indications for the use of central venous catheters
- difficult peripheral venous access
- delivery of certain medications or fluids
- medications such as vasopressors, chemotherapeutic agents, or hypertonic solutions that can damage peripheral veins
- additionally, catheters with multiple lumens enable the delivery of several parenteral medications simultaneously
- prolonged intravenous therapies that require a more stable and reliable access point
- specialized treatment
- hemodialysis, plasmapheresis, transvenous cardiac pacing, and invasive hemodynamic monitoring
Classification of intravascular access according to the expected time of insertion
| Classification of intravascular access according to the expected time of insertion |
► Short-term
► Long-term
|
Ventilation and respiration |
- maintain a patent airway and adequate oxygenation (O2 saturation ≥ 95%)
- SO2S trial showed no benefit from routine O2 administration ⇒ O2 administration is not recommended in nonhypoxic patients
- hypoxia (O2 saturation < 92-94%) should be corrected with supplemental oxygen 2–4 l/min via a nasal tube, and all possible causes of the hypoxia sought and treated (e.g., pulmonary edema, embolism, or infection)
- test breathing reflexes and regularly assess the risk of aspiration (many patients have bulbar or pseudobulbar syndrome) → see water swallow test below
- a common cause of acute respiratory insufficiency is aspiration and/or accumulation of mucus and saliva in the airways due to inadequate expectoration
- in cases of respiratory infection, administer antibiotics empirically after collecting sputum and swabs; adjust the therapy according to cultures and sensitivity results
- early intubation and ventilatory assistance may be necessary in the presence of a severely compromised respiratory pattern, severe hypoxemia or hypercarbia, and in unconscious patients (GCS ≤8)
- before intubation, consider the patient´s prior wishes, general condition, and prognosis
- Hyperbaric Oxygen Therapy (HBOT) is not recommended, except for stroke caused by air embolization (Murphy, 2019) (AHA/ASA 2019 III/B)
- limited data show no benefit
- HBO should be offered only in the context of a clinical trial or for individuals with air embolism
|
Signs of respiratory failure
|
|
ECG monitoring, cardiac care
|
Arrhythmia and other ECG changes
- acute stroke is associated with an increased risk of cardiac arrhythmias, affecting up to 25% of hospitalized stroke patients
- the incidence is greatest within the first 24 hours
- tachycardia is more common than bradycardia
- arrhythmias are more prevalent in patients with hemispheric lesions
- while some arrhythmias are benign (such as ventricular/atrial extrasystoles), persistent tachyarrhythmias may lead to hypotension or cardiac failure, potentially contributing to stroke progression
- atrial fibrillation should be excluded as a potential cause of the stroke
- ECG monitoring is recommended for ≥ 24-48 hours (AHA/ASA 2019 I/B-NR)
- especially in patients with previous known cardiac disease, unstable BP, signs/symptoms of heart failure, stroke involving the insular cortex
- ECG changes are common in acute stroke
- the ST segment and T wave are most commonly affected; these changes may mimic myocardial infarction (ST elevation can be present, but not depression) ⇒ exclude myocardial infarction in such cases
- the ST segment and T wave are most commonly affected; these changes may mimic myocardial infarction (ST elevation can be present, but not depression) ⇒ exclude myocardial infarction in such cases
- the situation is further complicated by frequent elevation in troponin levels, which is usually attributable to cerebral infarction rather than myocardial infarction
Cardiac output
- cardiac output should be optimized by maintaining a normal heart rate and a high normal BP
- low cardiac output may be caused by:
- dehydration (⇒ rehydration with IV fluids is necessary in patients who cannot swallow safely)
- heart failure, acute myocardial infarction
- inotropic agents may be required (dobutamine has the advantage of increasing cardiac output without substantially affecting heart rate or BP)
- cardiac dysrhythmias (⇒ drugs, cardioversion)
- the central venous pressure should be maintained at approximately 8–10cm H2O, but its monitoring is usually not necessary
Fluids and minerals
|
- monitor fluid balance every 6-24h and aim to maintain normovolemia or mild hypervolemia
- measure central venous pressure (CVP) if needed
- avoid fluid restriction, which increases the risk of worsening the ischemic deficit (especially in the case of subarachnoid hemorrhage)
- excessive volume replacement may provoke cardiac failure with pulmonary edema
- hypervolemic hemodilution and vasodilator therapy are not recommended
- a slightly negative fluid balance is recommended in patients with extensive brain edema
- initially, most patients require IV fluid replacement with normal saline
- hypotonic solutions (NaCl 0.45% or glucose 5%) are contraindicated (except when treating hypernatremia)
- monitor biochemical parameters (electrolyte levels, urea, creatinine, C-reactive protein, hepatic enzymes, and osmolality) as well as complete blood count (CBC) +coagulation profile
- adjust the frequency of sampling based on the patient’s condition and any detected abnormalities
- check acid-base balance
- acutely to detect conditions such as hypoxemia, hypercapnia, acidosis/alkalosis
- stable ventilated patients should be checked twice daily
Glycemia
|
- glucose serves as the primary energy source for the brain, with over 90% of the brain’s energy derived from the oxidation of glucose. Even during hypoglycemia, glucose remains the most important substrate for brain metabolism
- the brain/serum glucose ratio (typically 0.6-0.7) drops to 0.2-0.4 in cases of brain injury (resulting in increased sensitivity to hypoglycemia)
- no glucose solution should be given to a stroke patient unless hypoglycemia is detected
- monitor serum glucose levels in diabetic patients
Hyperglycemia
- hyperglycemia (stress hyperglycemia, stress diabetes) is present in up to 2/3 of stroke patients, worsening the outcome of all types of stroke and traumatic brain injuries (TBI)
- a meta-analysis focused on in-hospital mortality in critically with hyperglycemia showed that even mild hyperglycemia (6.1-8.0 mmol/l) in non-diabetics is associated with a 3.9-fold higher risk of death compared to completely normoglycemic individuals [Capes, 2000]
- according to the NICE-SUGAR study, the optimal target glycemia in intensive care is < 10 mmol/L (EUSI); hypercorrection to levels < 6 mmol/L increases mortality
- optimally maintain glycemia in the range of 7.8-10 mmol/L (140-180 mg/dL) (AHA/ASA 2019 IIa/C-LD)
- check glycemia every 6h (glycemic profile); more intensive monitoring is advised during continuous insulin administration
- use repeated boluses of subcutaneous insulin or continuous IV infusion → insulin protocol
- lower the raised blood glucose levels gradually and avoid hypoglycemia
- microdialysis studies indicate that intensive insulin therapy may lead to brain glucose deprivation and elevated levels of lactate and glutamate [Vespa, 2006]
Hypoglycemia
- hypoglycemia occurs less frequently and must be excluded in any stroke patient, as it may mimic the stroke
- significant hypoglycemia < 3.3 mmol (60mg/dL) must be corrected rapidly (IV dextrose bolus or infusion of 10–20% glucose) (AHA/ASA 2019 I/C-LD)
Fever
|
- maintain normothermia
- identify and treat sources of body temperature >37.5°C)
- causes of pyrexia:
- preceding infection which may be a risk factor for or cause of the stroke (endocarditis)
- the effects of the stroke itself (especially in SAH or ICH)
- a complication of the stroke (infection, VTE)
- administer antipyretic medication to hyperthermic patients (AHA/ASA 219 I/C)
- paracetamol 1000 mg every 4 hours
- the benefit of induced hypothermia is uncertain
Nausea and vomiting
|
- most commonly occurs in ICH, SAH, and brainstem ischemia
Prevention and management of GI complications
|
- gastrointestinal (GI) complications are common and significantly worsen morbidity and mortality
- most common complications:
- stress ulcer
- gastroesophageal reflux (GER)
- gastroparesis
- intestinal paralysis (paralytic ileus)
- ⇒ X-ray, abdominal SONO or CT
- endoscopic desufflation
- constipation/diarrhea
- singultus
- the most common cause of GI bleeding is either a preexisting lesion or a newly developed “stress ulcer”
- disruption of the integrity of the upper GI mucosa due to extreme physiological stress, typically in critically ill patients
- often develops within a few hours after the initial insult
- can result in bleeding or perforation ⇒ ↑ mortality and intensive care stay
- incidence approx. 3% when on prophylactic medication
- risk factors for GI bleeding
- coagulopathies, including iatrogenic
- history of GI bleeding/peptic ulcer
- mechanical ventilation > 48h
- traumatic brain/spinal cord injury
- sepsis
- corticosteroids use
- renal and hepatic impairment
- malignancy
- severe stroke
- initiate enteral nutrition as soon as possible!
- prophylaxis should be administered only to patients at increased risk and discontinued in a timely manner (due to the increased risk of nosocomial pneumonia, Clostridium difficile infection, drug interactions, or hepatotoxicity); routine use of PPIs does not reduce mortality
- proton pump inhibitors (PPIs)
- PPIs are more expensive and significantly more effective than H2-blockers [Buendgens, 2016]
- use H2 blockers if PPIs are contraindicated
- 40 mg once daily, or 20 mg twice daily PO
-
- 1g PO or via nasogastric tube every 6-8 hours
- used in peptic ulcer prevention and treatment or to reduce hyperphosphatemia
- caused by a clonic contraction of the diaphragm with simultaneous closure of the glottis
- short-term hiccups are mostly benign and can be attributed to factors such as:
- distention of the esophagus and stomach, intake of carbonated fluids, irritation of the digestive tract with spices
- emotions, excitement
- sudden change in temperature: drinks (hot/cold), shower, air, etc.
- more serious underlying causes:
- pulmonary and mediastinal diseases (pneumonia, lung tumors, mediastinitis, and mediastinal tumors)
- abdominal cavity diseases (direct irritation of the diaphragm – ileus, peritonitis, stomach and liver tumors and metastases, liver abscess, pancreatitis, and pancreatic tumors, etc.)
- heart diseases (pericarditis, myocardial infarction)
- esophageal diseases (oesophageal obstruction by solid food or tumor, or esophagitis)
- metabolic causes (uremia, diabetes decompensation), acid-base disorders, mineral imbalances (hyponatremia)
- central causes (direct or indirect brainstem lesions) – tumors, stroke, trauma
- alcohol and drugs (dexamethasone, methyldopa, sulfonamides, antiseizure medications)
- severe forms of hiccups are frequently resistant to symptomatic treatment
- treat potential causes
- pharmacotherapy (see table) – combination therapy may be effective (e.g., omeprazole + baclofen + gabapentin)
- psychotherapy (cognitive-behavioral therapy or other psychological interventions may be beneficial for stress-induced hiccups)
- acupuncture (may provide symptomatic relief, particularly for hiccups resistant to pharmacotherapy)
| BACLOFEN (has a peripheral and central effect) |
|
| Anticonvulsive drugs |
|
| gabapentin (NEURONTIN) |
|
| valproate (ORFIRIL, DEPAKINE) |
|
| Neuroleptics (central effect) | |
| HALOPERIDOL |
|
| chlorpromazine (PLEGOMAZIN) |
|
| Prokinetic drugs |
|
| metoclopramide |
PO 10 mg every 6-8 hours (max 40 mg/d) |
| PPI (use if GER is suspected) | |
| omeprazole pantoprazole |
PO 20-40 mg once daily |
Prevention and management of urinary complications
|
- bladder function should be assessed soon after stroke onset
- palpate the patient’s abdomen to detect a distended bladder
- perform an ultrasound of the bladder after a voiding attempt to assess the residual volume of urine in the bladder
- an indwelling catheter should be avoided if possible
- detrusor hyperreflexia causing urge incontinence and increased frequency of micturition
- incontinence of urine
- due to a combination of factors such as detrusor hyperreflexia, impaired sphincter control, preexisting prostatomegaly, immobility, inability to communicate, and urinary tract infection
- identify and manage the underlying cause and exacerbating factors
- catheterization increases the risk of trauma and infection
- urinary retention
- common, especially in men with preexisting bladder outflow obstruction, and must be systematically anticipated and excluded
- a urethral catheter provides prompt relief
- urinary infection
Pain management |
- pain is typically present in SAH, less commonly in ICH and ischemic stroke
- untreated pain may contribute to elevated blood pressure, tachycardia, or patient agitation
- rule out fractures or dislocations, which are often caused by falls due to sudden paresis
Dysphagia screening, oral hygiene
|
Nutrition
|
- catabolic state is common in the acute phase of stroke
- the total energy demand depends on the basic metabolic rate (BMR) and other factors
- early initiation of nutrition (within 24 hours) reduces the risk of various complications (malnutrition increases the risk of infection, muscle loss, etc.)
- enteral nutrition is preferred; if parenteral nutrition is necessary, use it for the shortest possible duration
- in patients with significant dysphagia, insert a nasogastric tube (NGS) for the prevention of aspiration bronchopneumonia
- follow oral hygiene protocols (AHA/ASA 2018 IIb/B-NR)
- Harris-Benedict formula – basal metabolic rate (BMR) [kcal/day]
- men = 66,47 + 13,75 x weight [kg] + 5 x height [cm] – 6,67 x age [years]
- women = 65,10 + 9,56 x weight [kg] + 1,85 x height[cm] – 4,68 x age [years]
- total energy demand = BMR x A factor x T factor (kcal/day)
- kJ = 4.18 * kcal
- for obese people (with a BMI > 30) prefer the Mifflin−St. Jeor (MSJ) equation → calculator
- men(kcal/day) = 5 + 10× weight (kg) + 6.25× height (cm) − 5× age (years)
- women(kcal/day) = −161 + 10× weight (kg) + 6.25× height (cm) − 5× age (years)
|
A factor
(activity)
|
immobile patient 1.2
|
|
|
mobile patient 1.3
|
||
|
T factor
(trauma)
|
surgery
|
minor-moderate 1.0-1.1
major 1.1-1.2
|
|
infection
|
light 1.0-1.2
moderate 1.2-1.4
severe 1.4-1.8
|
|
|
trauma
|
1.2-1.35
|
|
|
polytrauma
|
1.6
|
|
|
burn injury
|
< 20% 1.0-1.5
20-40% 1.,5-1.85
> 40% 1.85-1.95
|
|
Prevention of infection |
- adhere to barrier measures when in contact with the patient, ensure careful hand washing by staff
- follow protocols for the prevention of aspiration and early detection of impaired airway hygiene
- regularly evaluate the necessity of each invasive access, and replace them regularly
- risk of complications increases significantly:
- from day 3 for cannula
- from day 5 for urinary catheter
- from day 7 for central venous catheter (CVC)
- risk of complications increases significantly:
- conduct microbiological screening
- repeated sputum (throat and nasal swab) and urine cultures twice a week in the ICU
- take cultures after the patient’s transfer from another department
- isolate the patient if necessary (especially after transfers from another ICU, neurosurgery, etc.)
- repeated sputum (throat and nasal swab) and urine cultures twice a week in the ICU
- prophylactic administration of antibiotics is not indicated (AHA/ASA 2018 III/B-R)
Early rehabilitation and speech therapy |
- while complete recovery isn’t always possible, proper rehabilitation can help many stroke survivors regain function and improve their quality of life
- the effectiveness of rehabilitation depends on various factors, including stroke severity and location, timing and intensity of therapy ⇒ personalized, multidisciplinary approach(a comprehensive rehabilitation program) is crucial for optimal recovery
- immobile patients are at increased risk of:
- pneumonia
- decubitus ulcers (pressure sores)
- joint contractures (painful ‘frozen’ shoulder)
- deep venous thrombosis (DVT) and pulmonary embolism (PE)
- rehabilitation should start as soon as possible, including early verticalization
- however, overly aggressive therapy in the first 24 hours is not beneficial (AVERT study) (AHA/ASA 2019 III/B-R)
- the timing and intensity of rehabilitation after SAH and ICH must be individualized
Delirium, anxiety, depression
|
- search for signs of delirium, depression, or anxiety and treat them properly
- in patients without depression, fluoxetine therapy is not effective in enhancing poststroke functional status
→ Delirium
Preventing pressure ulcers |
- preventing pressure ulcers is crucial, especially since paralysis can lead to extended periods of bedrest, increasing the chance of pressure sore development
- pressure sores typically develop on the back of the head, shoulders, elbows, sacrum and buttocks, hips, and heels and cause considerable pain and slow the patient’s recovery
- preventive measures include:
Prevention of venous thromboembolism (VTE) |
- deep vein thrombosis is detected in the first 2 weeks in up to 50% of immobile patients [Brandstater, 1992]
Prophylaxis and management of acute symptomatic seizures |
- prophylactic administration of antiseizure medications (ASMs) to patients with recent stroke who have not had seizures is not recommended
- seizures should be managed according to standardized protocols.
→ see Acute symptomatic seizures
Brain edema, intracranial hypertension
|
→ Intracranial hypertension
→ Malignant cerebral infarction