ISCHEMIC STROKE / PREVENTION
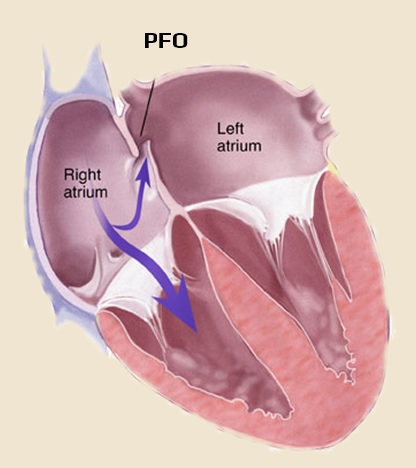
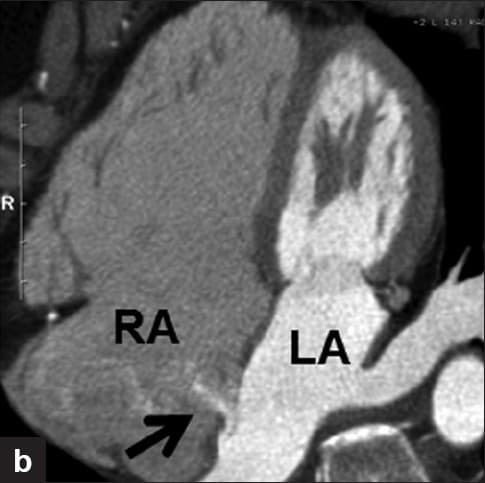
Patent foramen ovale (PFO)
Updated on 21/06/2024, published on 25/01/2023
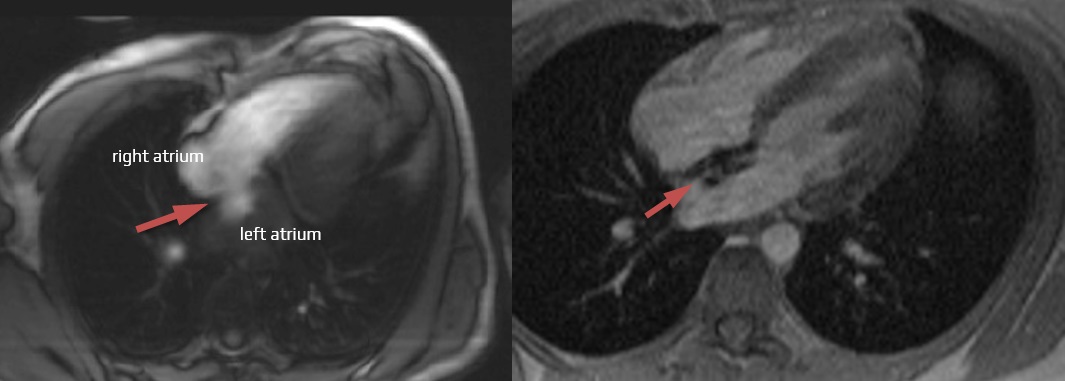
- before birth, the atrial septum is formed by two leaves; after birth, the pressure rises in the left atrium, and the two leaves fuse together
- in 1/3 of people, the leaves do not fuse, and a different-sized opening called a patent foramen ovale (PFO) or foramen ovale apertum (FOA) persist
- PFO may cause a right-to-left shunt, where blood can flow from the right atrium directly into the left atrium, bypassing the pulmonary circulation
- various particles in the blood can bypass the pulmonary circulation and enter the systemic circulation along with the blood (paradoxical embolism)
- small blood clots released from the veins of the lower limbs and pelvis
- gas bubbles (formed during rapid decompression in scuba divers or can be administered by IV injections)
- amniotic fluid
- fat
- certain hormones (serotonin in migraine with aura?)
- PFO alone does not cause hemodynamic problems
- PFO is not the only cause of right-to-left shunting:
- intracardiac shunts (90%) – patent foramen ovale (PFO), atrial septal defects (ASD), ventricular septal defects (VSD), truncus arteriosus
- extracardiac shunts (10%)
- mostly pulmonary AV shunts
- rare systemic to pulmonary artery fistulas (Livingston, 2019)
- persistent left superior vena cava (PLSVC)
- the PLSVC usually drains into the right atrium ( 80–90%) through a dilated coronary sinus but may drain into the left atrium (Tyrak, 2017)
- combination of extra- and intracardiac shunts is possible (Liu, 2020)
PFO and cryptogenic stroke
- patent foramen ovale (PFO) is associated with cryptogenic stroke (CS), though the pathogenicity of discovered PFO in the setting of CS is typically unclear
- the prevalence of PFO is ~ 25% in the general population and up to 46% in patients with CS
- PFO is occasionally associated with the following conditions:
- PFO-related stroke mechanisms:
- paradoxical embolization from peripheral veins
- embolization of a thrombus formed directly within the PFO canal
| Content available only for logged-in subscribers (registration will be available soon) |
Diagnostic evaluation
PFO detection
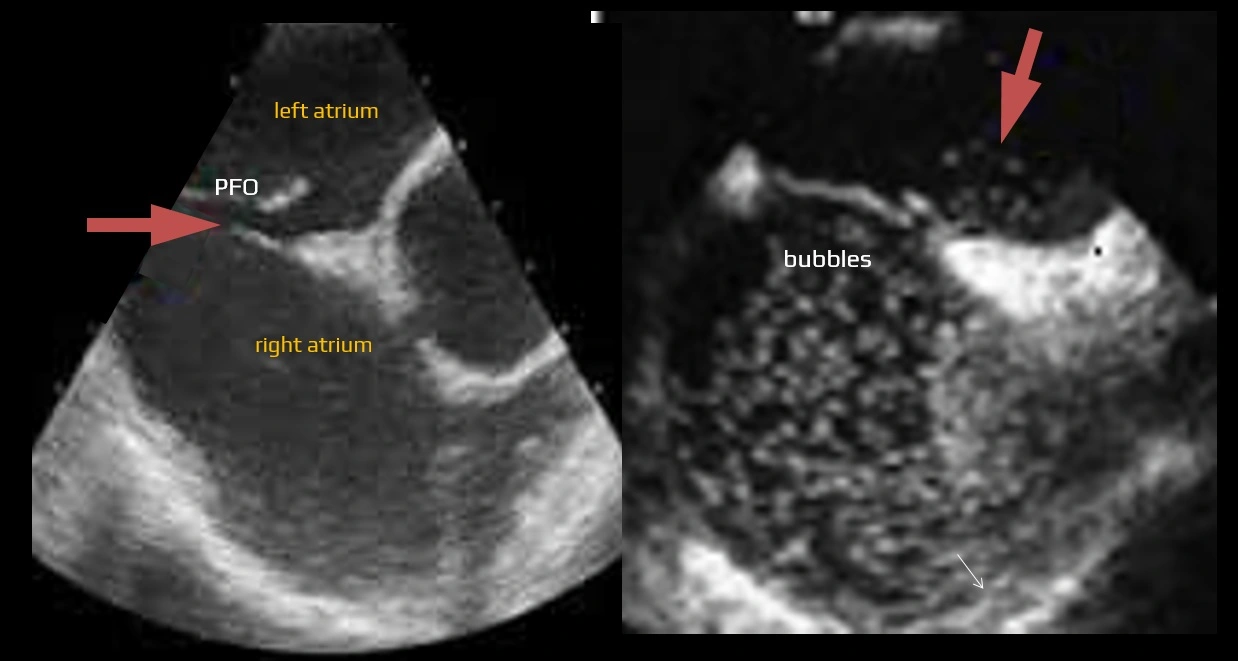
Contrast-enhanced transesophageal echocardiography (cTEE)
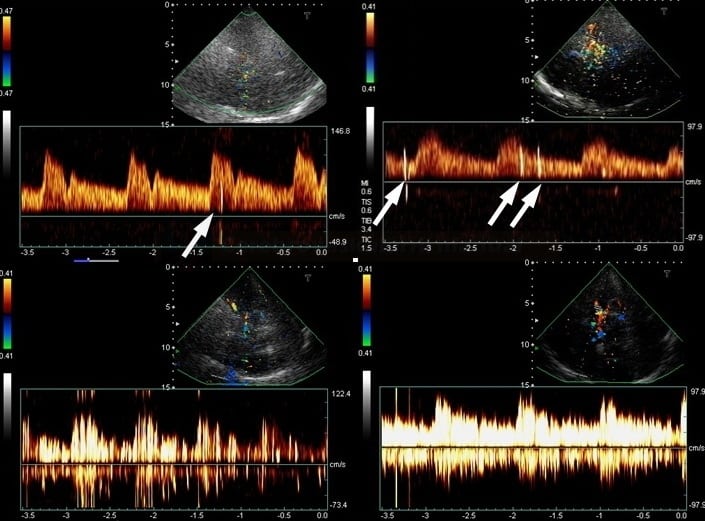
TCD/TCCD bubble test
- non-invasive screening method → see here
- detects all right-to-left shunts (cardiac and extracardiac)
- sensitivity and specificity reported over 90%
- the choice of vein used to introduce contrast (agitated saline in most cases) also influences the sensitivity for detecting a PFO
- increase in diagnostic sensitivity has been reported for a PFO when agitated saline contrast was injected via the femoral vein rather than the antecubital vein
- blood entering the right atrium via the inferior vena cava (IVC) is more directed towards the interatrial septum region where a PFO is found, as compared to blood entering the right atrium via the superior vena cava
Other methods
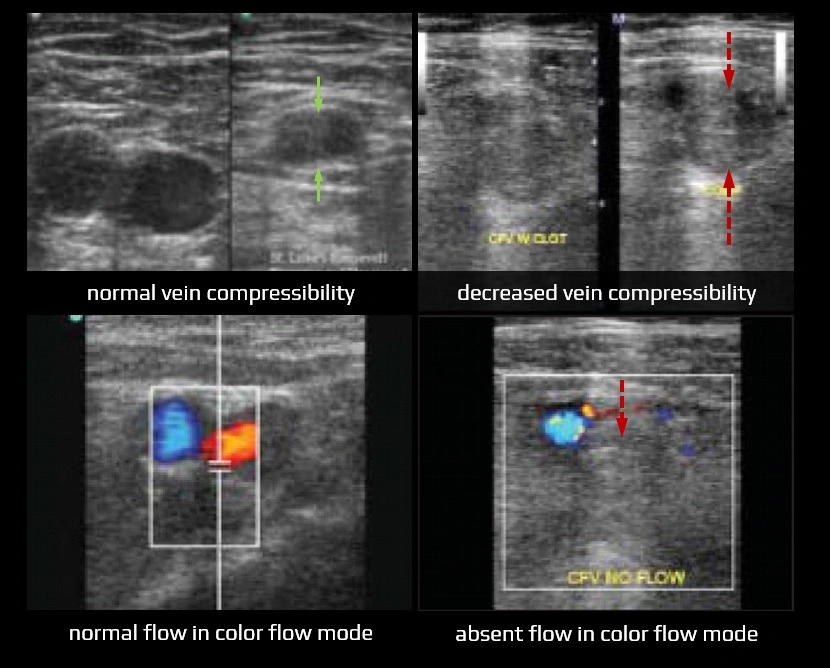
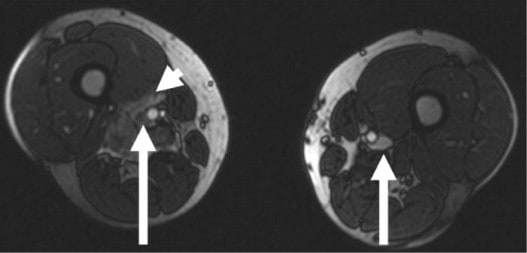
Doppler ultrasound / MR venography
- detection of a possible source of embolism in the veins of the pelvis and lower extremities
- issues regarding thrombosis detection are discussed here
D-dimers
- normal D-dimer levels: 0.068-0.494 mg/L
- leveles <0.5 mg/L make DVT unlikely but not impossible
- false-positive results are common
Management
- primary prevention: PFO closure not indicated
- secondary prevention: consider antiplatelet or anticoagulant therapy and PFO closure
| AAN 2020 | ESC 2018 | AHA/ASA 2021 |
| PFO closure in carefully selected patients (thorough evaluation must exclude more plausible etiologies) |
|
In patients with ESUS and PFO, closure may be considered, taking into account the likelihood of a causal role of the PFO. This should be a joint decision between the patient, the neurologist, and the cardiologist (I/C-EO) |
|
if the closure is not possible/available or is refused, anticoagulation is slightly preferred over antiplatelet therapy |
in patients 18-60 years of age with ESUS and high-risk PFO prefer PFO closure followed by antiplatelet therapy (2a/B-R) |
| in patients 18-60 years with ESUS and no high-risk PFO, the benefit of PFO occlusion versus antiplatelet therapy is not clearly established (2b/C-LD) | ||
| in patients 18-60 years with ESUS and PFO, there is no known benefit of PFO occlusion versus warfarin (2b/C-LD) | ||
| * different trials had different definitions (see table above) |
Conservative approach
- see table above – typically in patients with low-risk PFO, older age, and associated vascular risk factors in whom the causality of the PFO is highly uncertain
- antiplatelet therapy or anticoagulation (warfarin/DOAC) may be used as a conservative treatment (AAN 2020, Level C)
- the CLOSE trial was underpowered to demonstrate the superiority of anticoagulation over antiplatelet therapy
- a subanalysis of the NAVIGATE-ESUS trial showed a lower risk of stroke recurrence in PFO patients with rivaroxaban compared with aspirin [Kassner, 2018]
- according to the ESC guidelines 2018, anticoagulation may be considered in high-risk PFOs (large shunt or PFO with atrial septal aneurysm)

- in secondary prevention, a meta-analysis of randomized trials found that PFO closure had better outcome compared to medical therapy in large shunts
 [Ahmed, 2018]
[Ahmed, 2018] - AHA/ASA guidelines 2021 favor PFO closure in this setting (2a/B-R)
- in secondary prevention, a meta-analysis of randomized trials found that PFO closure had better outcome compared to medical therapy in large shunts
- if anticoagulation is indicated for another reason (e.g., VTE), the benefit of PFO closure is unknown ( AAN 2020, level B)
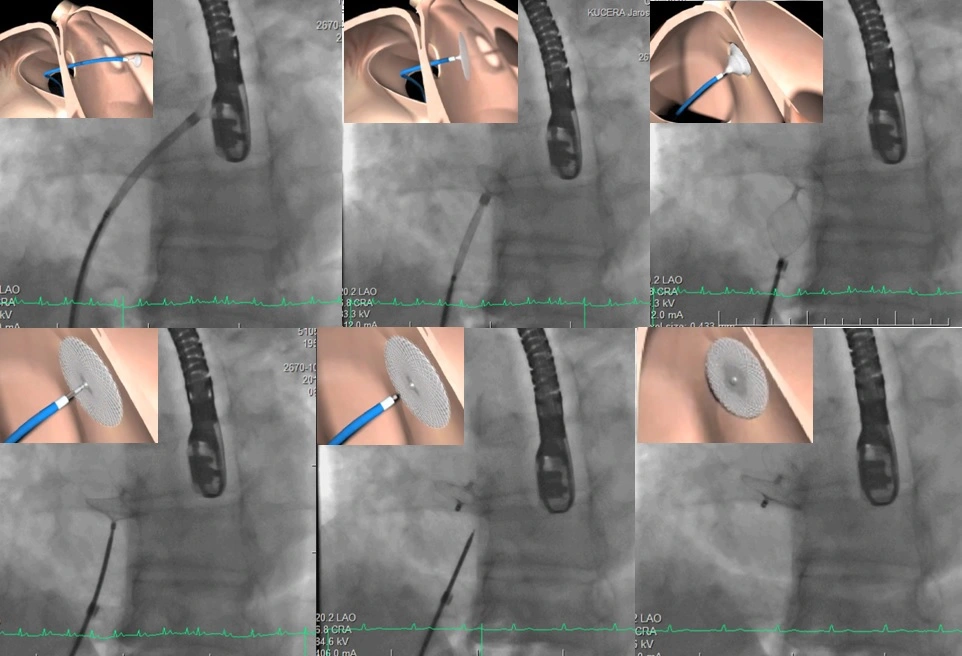
Endovascular procedure
- consider in carefully selected patients < 60 years of age with a high probability of paradoxical embolization (large shunt, embolic appearance of ischemia, high ROPE score)
- the benefit of closure is similar in the age groups < 45 years and 45-60 years
- closure may also be considered in patients aged 60-65 years without significant risk factors (AAN 2020, Level C)
- a multidisciplinary approach is advised (neurologist in collaboration with cardiologist + patient) (AHA/ASA 2021 1/C-EO)
- the vascular neurologist must assess the stroke mechanism and rule out other more likely etiologies!
- the size of the R-L shunt seems to be crucial for the indication; the presence of ASA is probably less important
- the patient must be informed about the benefits (absolute risk reduction 4.9%, NNT 20-29/5 years) and risks of the procedure (see below)
- there are different types of occluders (e.g., Amplatzer
 , STARflex
, STARflex  , Cardioform)
, Cardioform)
- Amplatzer was approved by the FDA in the fall of 2016 based on 10 years of data from the RESPECT trial
- Cardioform was approved by the FDA in 2018 based on data from the REDUCE trial → see here
- occluders are delivered percutaneously through the femoral vein into the right atrium and through the PFO into the left atrium
- occlusion is associated with the following risks:
- atrial fibrillation (even permanent!) – REDUCE 6.6%, CLOSE 4.6%! [Chen, 2021]
- thromboembolism
- perforation of the heart with hemopericardium
- air embolism
- device-related thrombosis (DRT)
- septal erosion
- residual shunt
-
an interesting alternative with a minimum of complications offers NobleStitch EL
Post-procedural management
- DAPT (ASA + clopidogrel) for 1-6 months, then monotherapy for ≥ 5 years [Pristipino, 2018]
- it is not clear whether antiplatelet drugs should be prescribed permanently or only temporarily (and for how long)
- even after successful occlusion, the incidence of stroke is not zero ⇒ other etiologies must be considered
- prevention of bacterial endocarditis for 6 months
- avoid activities with risk of large shocks or chest impact for 3 months
- TTE controls – no standard protocol: usually 3 – (6) – 12 months after the procedure
- a follow-up TCCD bubble test at 3 months is suggested after the procedure
PFO and migraine
- a higher prevalence of PFO has been reported in patients with migraine with aura than in patients with migraine without aura or patients without migraine (48% vs. 23% and 20%, respectively).
- the causal relationship remains unclear
- some small non-randomized trials have reported a reduced incidence of migraine after PFO closure
- randomized MIST (Starfix, 2016) and PREMIUM (Amplatzer, 2017) trials did not show a preventive effect
- in conclusion, it is less likely that PFO plays a role in the development of migraine headache
- the role of PFO in the development of ischemic stroke in migraineurs has not been determined yet
- patients with both migraine and stroke had larger shunts than patients with migraine without stroke, patients without migraine with stroke, and controls
- concerning the white matter hyperintensities (WMH), overall WMH did not differ by PFO presence; however, juxtacortical WMHs are more frequently found in patients with migraine and right-to-left shunting
- these findings suggest that incidental PFO may increase the risk of ischemic stroke in migraineurs