ISCHEMIC STROKE
Genetic Cerebral Small Vessel Diseases
Updated on 18/06/2024, published on 28/12/2023
- cerebral small vessel diseases (SVDs) encompass a group of disorders that primarily affect the small blood vessels in the brain (small arteries, arterioles, capillaries, and venules)
- typical SAVD features include leukoencephalopathy, lacunar strokes, and microbleeds
- the underlying causes of SVDs may differ:
- sporadic forms
- common in older populations, affecting approximately 80% of individuals aged 65 and nearly all aged 90, making it a prevalent age-related condition → Binswanger´s disease
- strongly associated with hypertension
- monogenic forms are rare
- responsible for 1%-5% of all strokes and several other disturbances (Manini, 2023)
- genetic discoveries have enabled a new etiology-based classification of SVDs
- sporadic forms
- both forms result in impaired blood flow, vessel wall damage, and leakage of blood components into brain tissues; these changes lead to significant morbidity and mortality due to stroke and dementia
- this article provides an overview of genetic SVDs, focusing on their etiology, clinical manifestations, neuroimaging features, diagnostic approaches, and management strategies
Pathophysiology
- the exact mechanisms underlying genetic small vessel diseases are diverse and depend on the specific genetic mutation
- mutations in genes encoding proteins involved in the structural integrity and function of cerebral small vessels
- commonly implicated genes include NOTCH3, HTRA1, COL4A1, and COL4A2
- these mechanisms may involve abnormal protein aggregation, inflammation, and damage to vascular smooth muscle cells and extracellular matrix, which collectively contribute to small vessel dysfunction and cerebral damage (ischemia or hemorrhage)
Clinical presentation
- despite many genes being involved, the phenotypes of monogenic cerebral SVDs partly overlap
- recurrent ischemic or hemorrhagic strokes, cognitive impairment, gait disturbances, and mood disorders
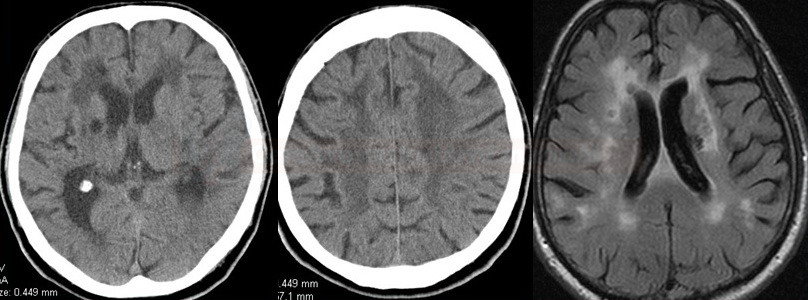
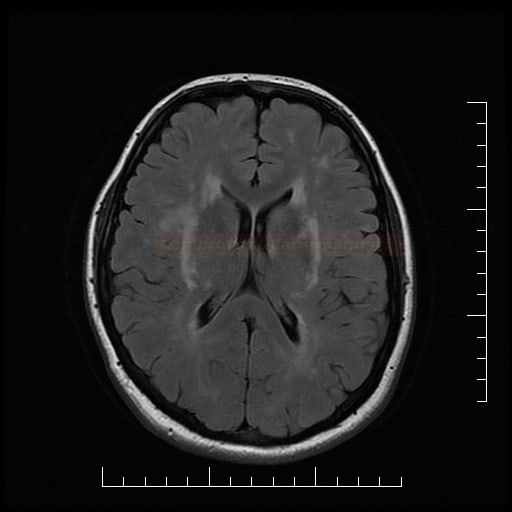
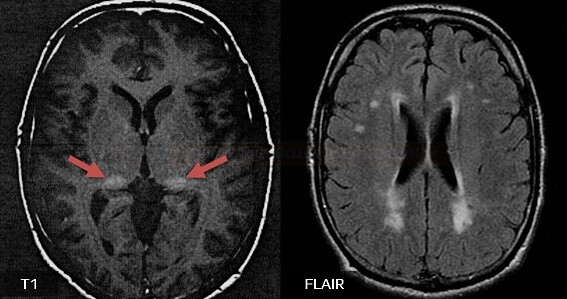
- lacunar infarcts and microbleeds and extensive white matter lesions (WMLs) are characteristic findings on neuroimaging
- the specific additional clinical features can vary depending on the underlying genetic mutation
- the severity and progression of symptoms vary depending on the specific genetic mutation and individual factors
Diagnostic evaluation
- combination of clinical evaluation, neuroimaging (MRI and CT scans), genetic testing to identify causative mutations
- MRI is crucial in identifying typical imaging features like white matter hyperintensities, microbleeds, and lacunar infarcts
- genetic testing confirms the diagnosis by identifying pathogenic mutations.
- genetic testing can be challenging and time-consuming – neurologists should have adequate knowledge about the genetic background of SVD to effectively select patients and choose the relevant genes for analysis
Management
- currently, there is no specific cure for most genetic small vessel diseases
- symptomatic therapy
- antiseizure medications (ASMs) for seizures, treatment of cognitive impairment and mood disorders
- renal monitoring with regular assessment of kidney function and treatment of kidney disease
- neurorehabilitation to improve mobility and cognitive function
- antiseizure medications (ASMs) for seizures, treatment of cognitive impairment and mood disorders
- risk factors modification – antihypertensive therapy, statins, and antiplatelet agents are commonly used to manage vascular risk factors
- preventing complications and providing supportive care
- family members may benefit from genetic counseling and screening
Overview of genetic disorders
Pure SVDs
| condition | inheritance | gene(s) | specific manifestations |
| CADASIL | AD | NOTCH3 | psychiatric disorders |
| CARASIL | AR | HTRA1 | alopecia, spondylosis |
| CARASAL | AD | CTSA | psychiatric disorders, cramps, migraine-like headache |
| RVCL-S | AD | TREX1 | cerebroretinal vasculopathy |
SVDs with additional involvement of large arteries
| condition | inheritance | gene(s) | specific manifestations |
| COL4-related angiopathies | AD | COL4A1 COL4A2 |
hemorrhages ophthalmologic malformations |
| Fabry disease | X-linked | GLA | neuropathic pain cataract renal and cardiac failure |
| homocystinuria | AR | CBS and others |
skeletal abnormities mental retardation |
CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy)
- one of the most well-known genetic small vessel diseases
- caused by mutations in the NOTCH3 gene
- NOTCH3 is a transmembrane receptor of the Notch family with a large extracellular domain (ECD) mainly consisting of epidermal growth factor (EGF)-like repeats
- more than 230 pathogenic NOTCH3 mutations have been described
- characterized by recurrent strokes, cognitive decline, depression, and white matter abnormalities on brain imaging
- symptoms appear 10-15 years earlier in patients with CARASIL
CARASIL (Cerebral Autosomal Recessive Arteriopathy with Subcortical Infarcts and Leukoencephalopathy)
- CARASIL is a rare AR disorder associated with mutations in the HTRA1 gene
- it leads to similar symptoms as CADASIL, including strokes and cognitive impairment
- specific features include: (Fukutake, 2011)
- the recessive mode of inheritance
- early age of onset (20 to 30 years)
- the frequent occurrence of spondylosis and alopecia as associated non-neurologic manifestations
- typically an absence of migraine and depression
Fabry disease
- caused by mutations in the α-galactosidase A (α-Gal-A) gene located on the X chromosome
- phenotype varies depending on the specific gene defect and residual enzyme activity; the severe form may be seen in both males and heterozygous females
Familial CAA (Cerebral Amyloid Angiopathy)
- caused by mutations in the APP, CST3, or ITM2B genes inherited in an AD pattern (one copy of the mutated gene is sufficient to cause the disease)
- the Dutch type (the APP gene mutation) is the most common, with stroke often presenting as the initial symptom
- characterized by the accumulation of amyloid protein in the blood vessel walls of the brain, leading to bleeding and cognitive deficits
- individuals with hereditary CAA experience progressive deterioration of cognitive functions (dementia), stroke, and other neurological complications beginning in mid-adulthood
RVCL-S (Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic Manifestations)
- a rare AD disorder resulting from mutations in the TREX1 gene
- the dysfunction of TREX1 leads to a complex pathology primarily affecting small blood vessels in the brain and retina (cerebroretinal vasculopathy)
- it can lead to strokes and cognitive decline, vision loss (due to retinopathy), and other systemic manifestations (renal impairment, liver dysfunction, and Raynaud’s phenomenon)
→ Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic manifestations (RVCL-S)
CARASAL (cathepsin A-related arteriopathy with strokes and leukoencephalopathy)
- ultra-rare monogenic autosomal dominant (AD) condition caused by point mutations in the CTSA gene (chromosome 20q13.12), which encodes the enzyme cathepsin A; these mutations lead to abnormal blood vessel development and function in the brain (particularly affecting the white matter and deep brain structures)
- cathepsin-A is a carboxypeptidase that associates with the lysosomal enzymes b-galactosidase and neuraminidase, promoting their stabilization
- CARASAL usually becomes apparent in adulthood, typically during the third to fifth decades of life
- clinical presentation: (Finsterer, 2019)
- stroke
- migraine-like headache
- cognitive impairment and behavioral disinhibition
- depression
- movement disorders, e.g. gait disorder, dystonia
- facial pain
- muscle cramps
- therapy-resistant hypertension (Bugiani, 2017)
- MRI scans typically show evidence of small subcortical infarcts (basal ganglia, thalamus) and diffuse extensive white matter changes, sparing temporal white matter
- microbleeds are rare
COL4 disorders
- COL4A disorders refer to a spectrum of genetic conditions resulting from mutations in the COL4A1 and COL4A2 genes
- these genes encode one of the collagen proteins, specifically collagen type IV, a critical component of basement membranes in various tissues, including blood vessels, kidneys, and the brain
- mutations in these genes lead to multisystemic manifestations, predominantly affecting the cerebrovascular system, eyes, and kidneys
- Pontine Autosomal Dominant MicroAngiopathy with Leukoencephalopathy (PADMAL) is an autosomal dominant monogenic belonging to COL4A1-related disorders (Roos, 2023)
- HANAC syndrome (hereditary angiopathy with nephropathy, aneurysms, and muscle cramps) (Alamowitch, 2009)
- Pontine Autosomal Dominant MicroAngiopathy with Leukoencephalopathy (PADMAL) is an autosomal dominant monogenic belonging to COL4A1-related disorders (Roos, 2023)
- the effects of COL4A1 mutations can vary widely among individuals, and the severity of symptoms can differ
Clinical presentation
- neurological: cerebral hemorrhages, ischemic strokes, and white matter abnormalities. Patients may present with seizures, migraines, and cognitive deficits.
- ocular: anterior segment dysgenesis, retinal arteriolar tortuosity, and cataracts
- renal: hematuria, chronic kidney disease, and in some cases, renal failure
Diagnostic evaluation
- neuroimaging (MRI) often reveals characteristic features such as white matter lesions, microbleeds, and cerebral hemorrhages
- genetic testing confirms mutations in COL4A1 or COL4A2 genes