ISCHEMIC STROKE / ACUTE THERAPY
Mechanical recanalization
David Goldemund M.D.
Updated on 11/06/2024, published on 26/11/2023
Introduction
- mechanical recanalization (thrombectomy or endovascular treatment) is an essential method for treating large vessel occlusions (LVO)
- the goal is to achieve rapid and complete recanalization ⇒ TICI 2b/3 (AHA/ASA 2019 I/A)
- mechanical treatment is performed after preceding IVT (bridging therapy) or as direct thrombectomy (dMT) if IVT is contraindicated (ESO-ESMINT 2019)
- intravenous thrombolysis (IVT) should not be skipped before thrombectomy in eligible patients
- IVT+MT is preferred over IVT alone
- the ESO guidelines 2023 recommend tenecteplase 0.25mg/kg over alteplase 0.9 mg/kg for patients scheduled for thrombectomy
- intravenous thrombolysis (IVT) should not be skipped before thrombectomy in eligible patients
- start treatment ASAP!
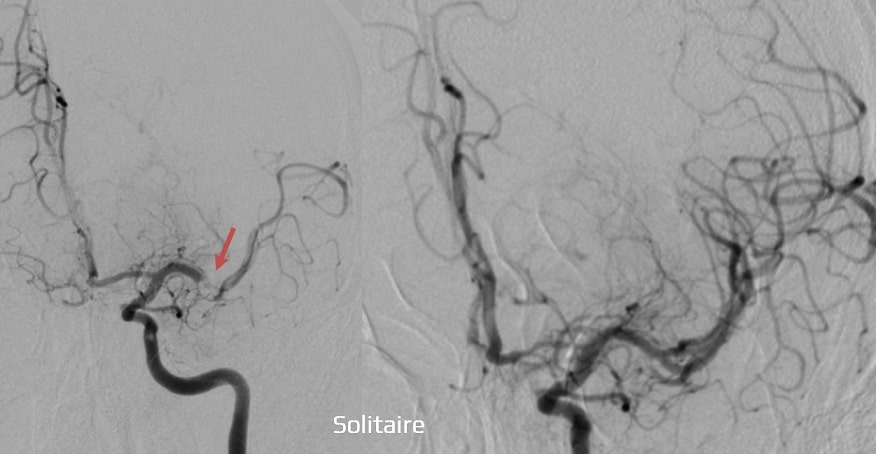
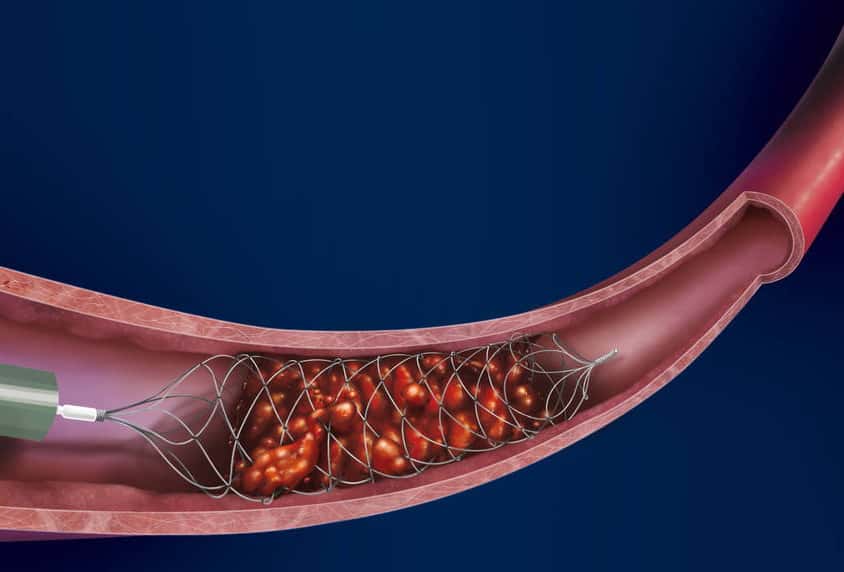
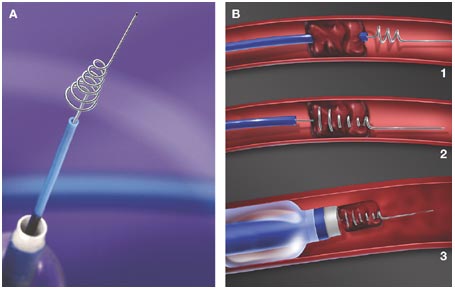
- stent retrieval technique (SR) alone, aspiration, or combined retrieval–aspiration techniques are used (according to the ASTER and COMPASS trials, the efficacy is comparable)
Time ≤ 6 hours from onset of symptoms
- the effectiveness of MT (EVT) performed ≤ 6h from the onset of stroke was demonstrated by trials using retrievers (published in 2015)
- EXTEND-IA, ESCAPE, SWIFT PRIME, THRACE, and REVASCAT
- trials showed high efficacy (recanalization rate up to 90%) and low risk of sICH (MR CLEAN 6%, ESCAPE 3.6%, EXTEND IA 0%, REVASCAT 1.9%)
- the absolute effect of treatment in achieving patient independence is 14-31%!
Time > 6 hours from onset of symptoms
| Content available only for logged-in subscribers (registration will be available soon) |
| Content available only for logged-in subscribers (registration will be available soon) |
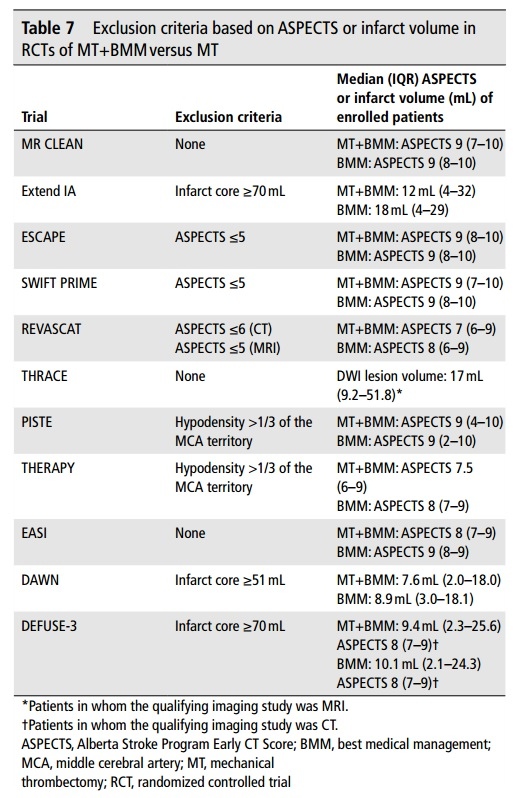
- randomized trials SELECT2 (2023), ANGEL-ASPECT (2023), and RESCUE-JAPAN LIMIT trial (2022) demonstrated a significant effect of MT in patients with large infarcts
- ASPECTS 3-5 or core (CBF/ADC) ≥ 50 mL (ASPECT-ANGEL)
- an update of guidelines can be expected soon
- thrombolysis facilitates the effect of mechanical thrombectomy
- the MR-CLEAN NoIV, SWIFT-DIRECT, and DIRECT-SAFE trials failed to show the superiority of dMT
- several meta-analyses have shown better outcomes with combination therapy compared to dMT [Vidale, 2020] [Katsanos, 2019] [Wang, 2020]
- IVT led to peripheral thrombus migration in 54% of patients in the INTERRSeCT trial, with 27% experiencing significant thrombus shift, which was associated with a better outcome. Another 27% had a moderate shift with no effect on the outcome [Ohara, 2020]
- sometimes, thrombus displacement to a more peripheral location may not lead to clinical improvement and can make subsequent endovascular procedures difficult or impossible [Flint, 2020]
- thrombolysis should not significantly delay the initiation of MT → do not wait for its effect and immediately transfer the patient to thrombectomy center (ESO-ESMINT 2019, AHA/ASA 2019)
- the RACECAT trial should answer whether it is better to thrombolyse the patients in the Stroke Center and then transport them to the Comprehensive Stroke Center (“drip and ship”) or transport the patient directly to the CSC (“mothership”)
Overview of mechanical recanalization methods
|
Methods rarely used or obsolete :
|
Advantages and disadvantages of MT
- a wider therapeutic window
- high potential for recanalization
- ability to treat patients who cannot undergo intravenous thrombolysis due to major contraindications (e.g., after recent surgery, during anticoagulation therapy) or where IVT has failed
- effective in thrombolysis-resistant occlusions
- can be used in combination with other revascularization procedures (IVT + IAT + mechanical recanalization)
- results highly depend on the skill and experience of the neurointerventionalist
- high costs and requirements for instrumentation and specialized personnel
- delayed initiation of therapy compared to IVT
- complications associated with endovascular procedures (dissection, perforation, etc.)
- risk of thrombus fragmentation leading to multiple distal embolizations
Indications
| The CTP selection discussed below refers to anterior circulation strokes. BAO can be treated within 24 hours without CTP selection |
| Acute ischemic stroke with a significant deficit and intracranial artery occlusion (occlusion detected by CTA, MRA, or TCCD)
|
|
Time window 0-6 hours
|
|
|
Time window 6-7 hours 18 minutes
|
|
| Time window 16-24 hours |
|
| Time window 6-24 hours + low ASPECTS |
|
- patients with low ASPECT score – TENSION, IN EXTREMIS (LASTE part)
- patients with minor deficit – IN EXTREMIS (MOSTE part), ENDOLOW
- concomitant neuroprotection
- overall negative results of the ESCAPE-NA1 trial with nerinetide (NA1); some benefit was seen in patients without prior IVT (interaction of NA1 with alteplase?) → see here
- IVT in patients with basilar artery occlusion during an extended time window – POST-ETERNAL
Contraindications
Contraindications are derived from individual trials with thrombectomy, which may differ slightly from each other.
Imaging findings
- impossibility to reach the target vessel (usually relative CI) → Unfavorable vascular anatomy during endovascular treatment
- absent penumbra and large early ischemia
- relative contraindication – even patients with extensive early ischemia (ASPECTS 3-5) may achieve a better outcome after MT compared to the conservative management, as shown in SELECT2 (2023), ANGEL-ASPECT (2023), and RESCUE-JAPAN LIMIT trial (2022)
- benefit in patients with ASPECT 0-2 is not yet proven
- cerebral vasculitis
- malignant intracranial tumor – no data on MT is available in patients with intracranial tumors. It is not recommended to perform MT in patients with intra-axial and metastatic tumors
Personal history, laboratory and clinical findings
- pregnancy is a relative CI
- relevant data on thrombectomy in pregnant women are not yet available; the risk of serious complications and risk-benefit ratio must be considered
- relevant data on thrombectomy in pregnant women are not yet available; the risk of serious complications and risk-benefit ratio must be considered
- age
- age < 18 years is not a contraindication; MT may be beneficial
- advanced age is not a contraindication to MT; even patients ≥ 85 years may benefit from it (Mc Donough, 2022)
- expected survival < 3 months (not because of cerebral infarction)
- intra-axial and metastatic tumors (assess individual prognosis)
- allergies
- history of severe allergic reaction to contrast agent (relative CI)
- allergy to nickel or titanium!
- premorbid mRS > 3 points (relative CI)
- NIHSS > 25 points (relative CI)
- thrombocytopenia is not a CI
Anticoagulant therapy
- using anticoagulants is not a contraindication to mechanical recanalization; the benefit is clear for large vessel occlusions → see here
Interventional radiologist consultation
During the consultation, the interventional radiologist needs to know (unless they have immediate access to the neuroimaging):
- severity of neurological deficit and current NIHSS
- ASPECT score ( important thresholds are 6 and 3 points, respectively)
- patency of the proximal (access) arteries → CT angiography
- tandem extra- and intracranial occlusion?
- is the extracranial occlusion acute? what is the extent of the occlusion (whole ICA or just terminal segment?)
- quality of collateral circulation
- presence of unfavorable anatomical conditions
- localization and extent of intracranial occlusion (segment, ev. clot burden score)
Thrombectomy procedure
Periprocedural monitoring
- the procedure is performed by a neurointerventionalist and supervised by an anesthesiologist and/or neurologist
- MT is performed in general anesthesia (GA) or conscious sedation (CS)/analgosedation
- this decision is made individually, with a preference for CS
- the GOLIATH, ANSTROKE, and SIESTA trials did not indicate a worse outcome with GA compared with CS
- data from an Italian registry show worse outcomes for patients treated under GA [Cappellari, 2020]
- disadvantages of GA:
- difficulty in monitoring neurostatus
- delay in the initiation of therapy
- a higher risk of periprocedural hypotension
- monitor BP every 5 minutes and continuously monitor pulse and oxygen saturation
- maintain BP < 180/105 mmHg during and after the procedure (for 24h) (AHA/ASA 2019 IIa/B-NR)
- according to the analysis of MR CLEAN data, optimal SBP appears to be ~ 135 mmHg, and the mean BP during MT should be ~ 150 mmHg → see here
- RIGHT-2 and MR ASAP trials are currently ongoing
- a repeated bolus of midazolam (2.5mg) + sufentanil (25ug) is recommended for sedation
- regularly check neurological status (level of consciousness, speech, visual field, motor skills)
Periprocedural anticoagulation
- dilute heparin with NS – Heparin 4ml (1mL=5000IU) + NS 16mL (⇒ 20mL of solution = 20000 IU of heparin)
- initial bolus injection
- 2000 IU of undiluted heparin (0.4 mL) or 30 IU/kg
- skip the bolus if thrombolysis preceded the procedure
- continue with a diluted heparin (20mL=20 000jj)
- at a dose of 500 IU/h (0.5mL/h) (PROACT trial protocol)
- at a dose of 450 IU/h (SWIFT PRIME trial protocol)
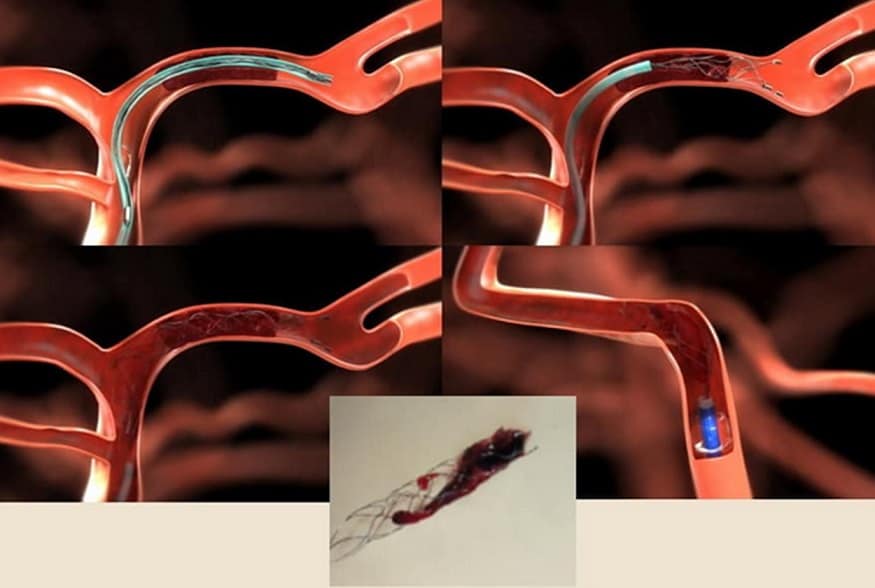
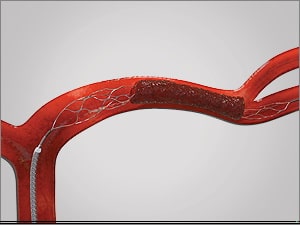
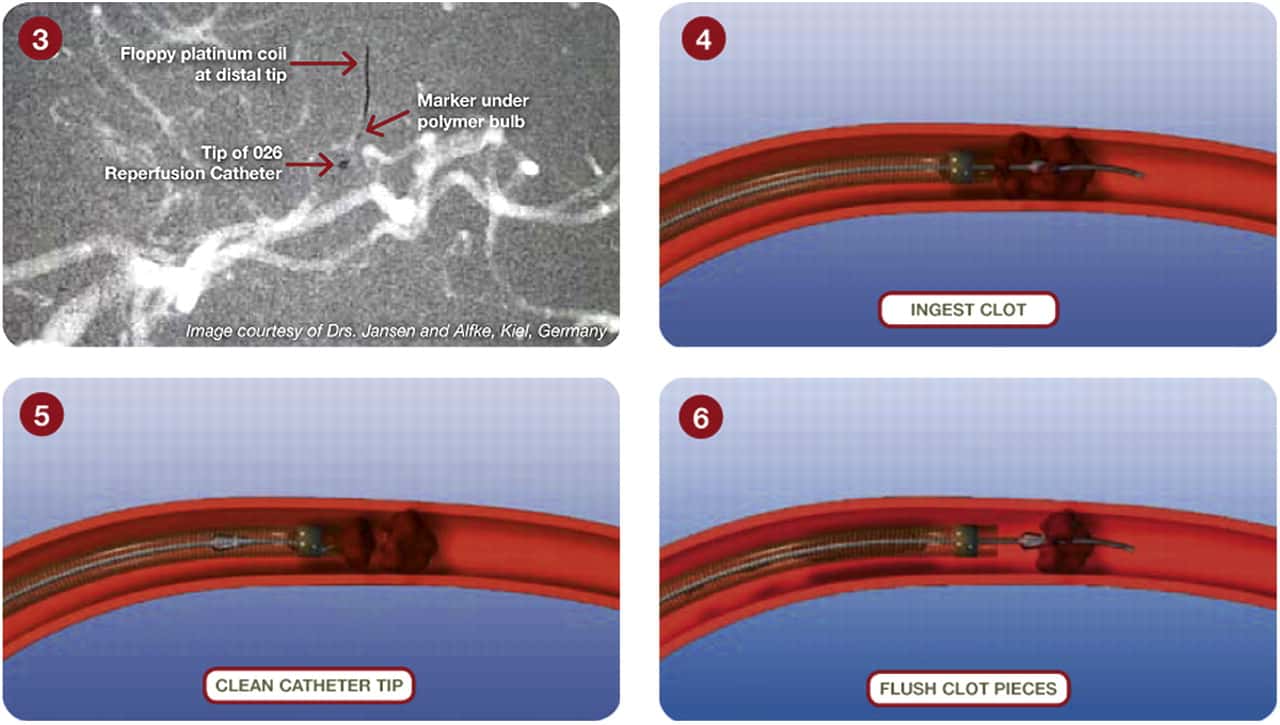
Thrombus extraction
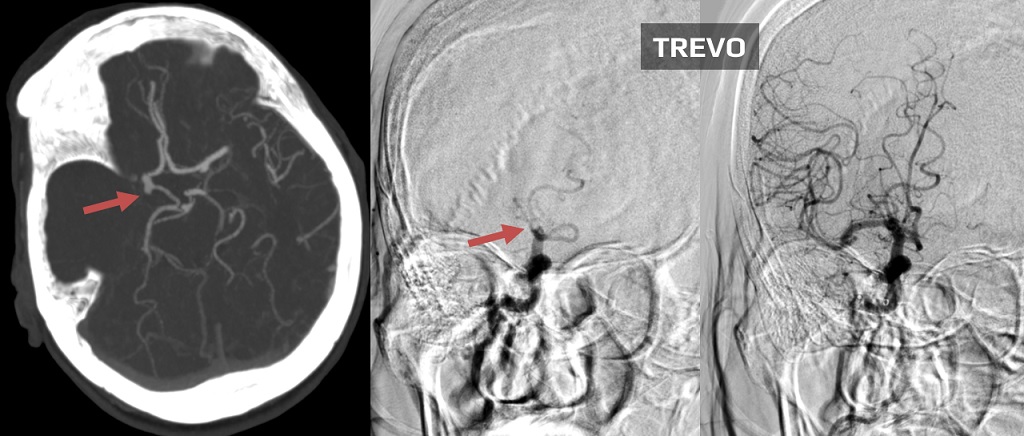
- initial assessment of the access route, location, and extent of occlusion and collateral circulation
- after passing the catheter through the thrombus, assess the patency of the distal segments; then, thrombectomy is initiated
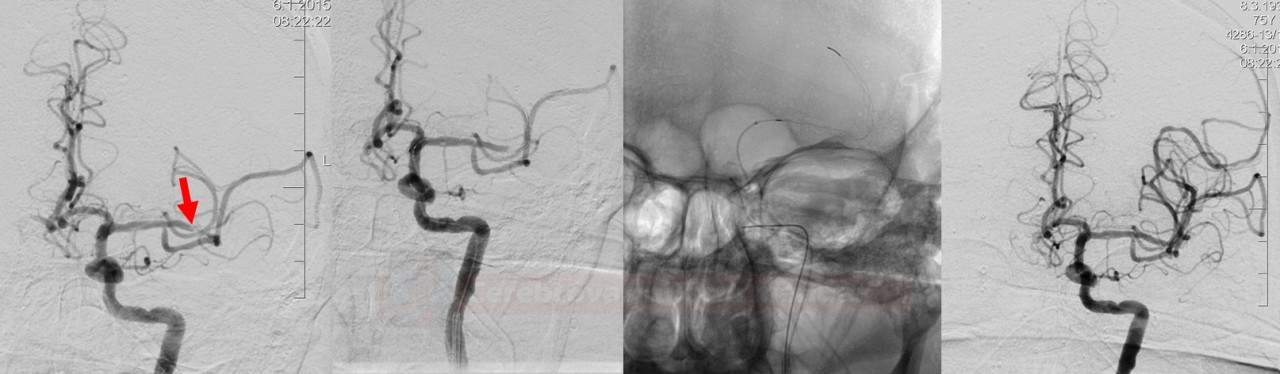
- perform follow-up angiographic controls after stent passage and at the end of the procedure
- if severe stenosis or occlusion of the proximal ICA is present, acute angioplasty with/without stenting can be performed
[Cohen,2015] (AHA/ASA 2019 IIb/B-R)
- fresh occlusion can usually be easily passed through with the guiding wire
- thrombus extraction followed by carotid angioplasty seems to give better results → see here
- subsequent antiplatelet therapy (contraindicated after IVT) is problematic if the stent is inserted
- in addition, moving wires through the stented segment may lead to local complications
- the goal of the procedure is to achieve a TICI of 2b-3
- the procedure can be combined with the administration of intra-arterial thrombolysis (IAT) (AHA/ASA 2019 IIb/C-LD)
- to treat inaccessible segment thrombosis or distal embolizations [Kaesmacher, 2019]
- good clinical effect of MT with TICI 2a-3 followed by IAT was reported in the CHOICE trial; the effect can be explained by the dissolution of microthrombi in the peripheral circulation
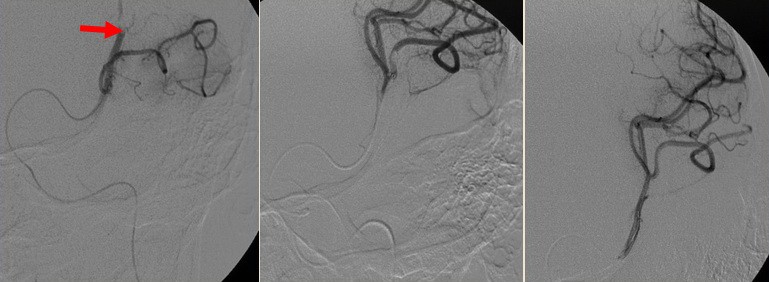
- if recanalization is achieved, wait ~ 15 minutes and perform a control examination to rule out early reocclusion
- with residual occlusions in distal segments, consider the benefit and risk of complications of the continued procedure
- if recanalization is not achieved, the procedure is terminated:
- after repeated unsuccessful extraction attempts (approximately 6 attempts)
- when sICH is suspected
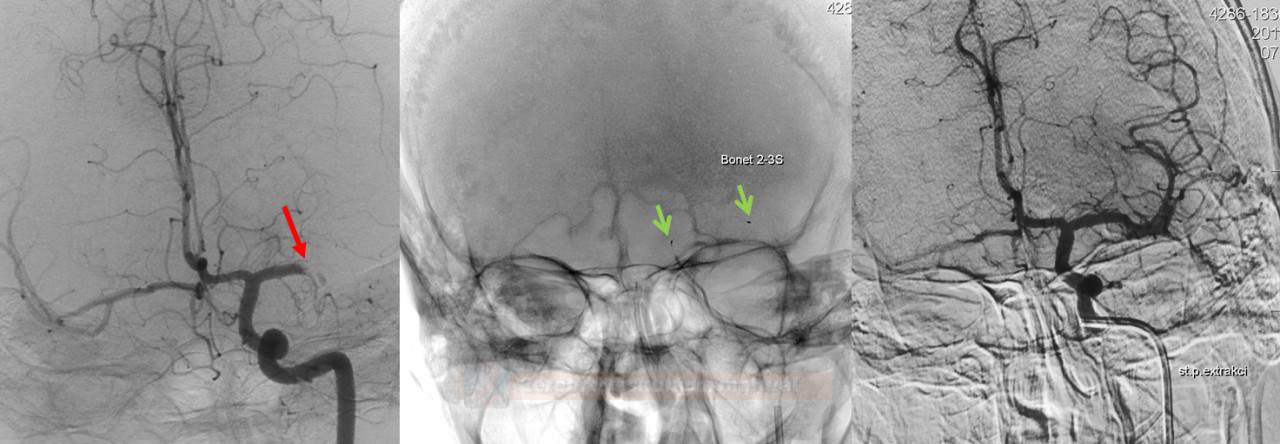
- after successful extraction, seek Early Venous Filling (EVF) at follow-up injection
- EVF is defined as the filling of any vein earlier than the late arterial phase (Elands, 2021)
- EVF is a marker of hyperperfusion and increased risk of hemorrhagic transformation
- EVF is assessed simply as present or absent
| Quality indicators of the neurointervention program |
|
Postprocedural care
- the sheath is extracted in the angio suite, and the entry is secured with an occluder (Perclose, Mynxgrip, AngioSeal )
- the patient is transported to the ICU; if SAH or ICH is suspected, a repeat CT scan should be performed
- in the ICU, continue close monitoring of vital signs (BP every 15min for the first hour, then every 30 minutes for at least 24 hours)
- maintain BP < 180/105 mmHg (AHA/ASA 2018 IIb/B-NR)
- the optimal threshold for SBP remains to be determined ( ∼ 140-180 mmHg) and may vary from case to case
- conflicting results have been published:
- aggressive BP treatment (< 130/80 mmHg) may be beneficial
[Choi, 2019]
- other data show that using an antihypertensive drug to target SBP < 160 mm Hg or 140 mm Hg may not be beneficial (MEDSCAPE)
- aggressive BP treatment (< 130/80 mmHg) may be beneficial
- the optimal threshold for SBP remains to be determined ( ∼ 140-180 mmHg) and may vary from case to case
- monitor neurological status (NIHSS, GCS) and search for complications
- if bridging therapy with tPA was used:
- monitor for bleeding (puncture, gingival, GIT, urogenital bleeding, etc.) and angioedema → see protocol of IV thrombolysis
- NG tube and central venous catheter placement and arterial puncture should be avoided in the first 24 hours
- IM injections should not be administered for ~ 1h after the thrombolysis initiation
- check CBC + coagulation tests (aPTT, TT, INR) at 6 and 12 hours, CBC+coagulation tests+metabolic panel the next day
- monitor for bleeding (puncture, gingival, GIT, urogenital bleeding, etc.) and angioedema → see protocol of IV thrombolysis
- perform a follow-up CT scan within 22-36 hours
Postprocedural antithrombotic medication
- the antithrombotic regimen after direct mechanical recanalization is not clearly defined in guidelines; there is no clear contraindication to ASA within 24 hours after dMT
- in the ESCAPE trial, ASA 160 mg was administered immediately after the initial CT scan
- with bridging therapy (IVT+MT), antiplatelet therapy, and mini-heparinization (as DVT prophylaxis) may be started after 24 h (after the exclusion of hemorrhagic complications on CT) → see Intravenous thrombolysis
- ASA can be given within 24 hours if a significant benefit is expected (e.g., after stenting) (AHA/ASA 2019 I/A)
- there is no standardized antithrombotic protocol for emergent stenting in thrombolyzed patients
- consider the risk of bleeding vs. the risk of stent thrombosis – it may be better to perform acute angioplasty with delayed stenting (on appropriate antithrombotic therapy)
- if a stent is implanted, it is necessary to start antithrombotic treatment immediately; various protocols can be found in the literature (not yet specified in guidelines)
- aspirin 500mg IV bolus given immediately after the procedure + clopidogrel added after 24h [Panpangiotou, 2011]
- abciximab (INTEGRILIN) IV bolus + continuous IV infusion for 12 hours, followed by ASA+CLP [Wang, 2007]
(Integrilin) usually 1mL/2mg or 1mL/0.75 mg
- IV bolus: 0.2 mg/kg within 5 minutes, followed by infusion (Sedat, 2014)
- IV infusion: 5mL(10mg) + 45mL of NS (1mL=0.2 mg) …… 3mL/h (0.6 mg/h) 0.125ug/kg/min (max 10ug/min!)
- IA bolus: 5mL /10mg) + 45 mL of NS (1mL=0.2 mg) …… bolus 10 mL (2mg) every 5 minutes till max dose 10 mg
(Reopro) 1mL/2mg
- a monoclonal anti-glycoprotein IIb/IIIa receptor antibody
- IV bolus: 0.25 mg/kg within 5 minutes, followed by infusion
- IV infusion: 5mL (10mg) + 45mL NS (1mL of solution = 0.2 mg) …… 3mL/h (0.6 mg/h) 0.125ug/kg/min (max rate 10ug/min!)
- IA bolus: 5mL (10mg) + 45 mL NS (1mL of solution = 0.2 mg) …… bolus 10 mL (2mg) every 5 minutes, max dose 10mg