ISCHEMIC STROKE / CLASSIFICATION AND ETIOLOGY
Etiologic classification of ischemic stroke
Updated on 24/03/2024, published on 19/04/2023
- individualized stroke prevention is based on the presumed underlying etiology ( patients with symptomatic high-grade carotid stenosis can be treated with CEA, while patients with cardioembolic infarcts caused by AFib are treated with anticoagulants)
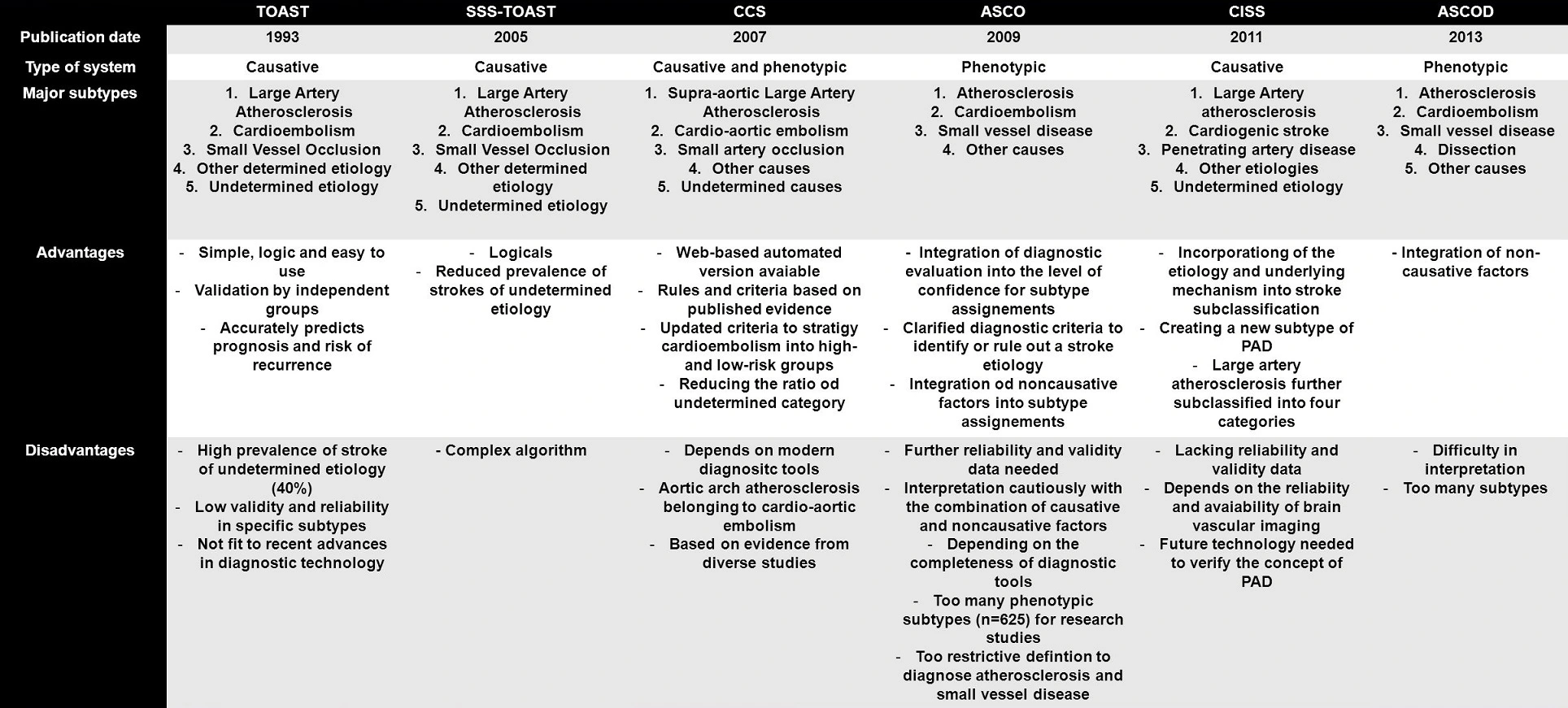
- the use of standardized diagnostic algorithms and established classifications (such as TOAST and CISS) is recommended for accurate diagnosis and treatment
Evaluation of ischemic stroke etiology
Brain imaging
- assess the nature, size, and location of the lesion on brain CT/MRI scans; note any other pathological findings
- both CT and MRI are commonly used in the acute stroke setting
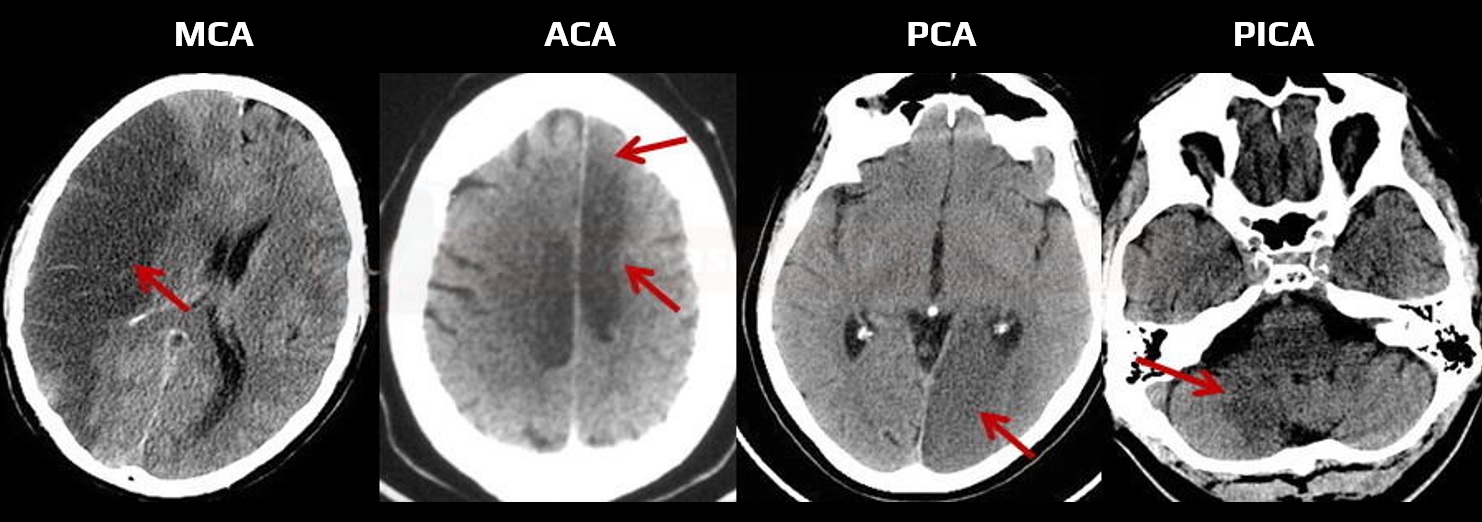
- based on the affected arterial territory, a specific artery occlusion can be assumed
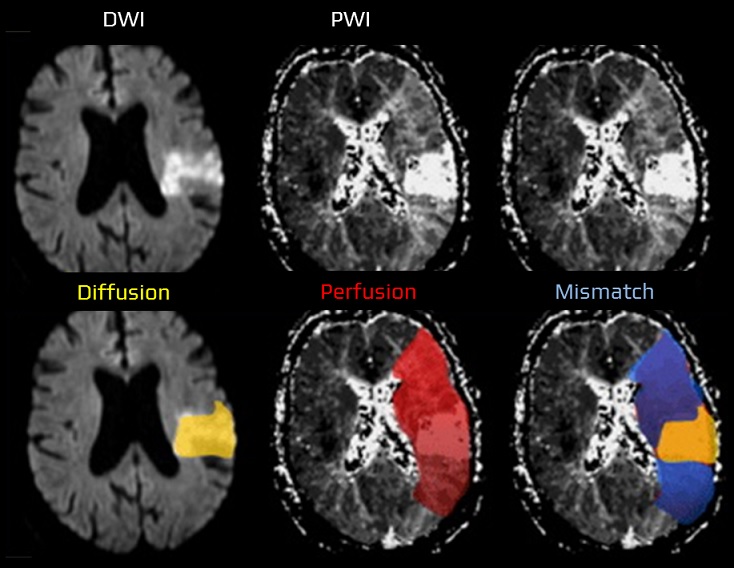
- it is reasonable to perform follow-up imaging for patients with suspected acute stroke and a negative baseline CT/MRI to confirm the diagnosis (AHA/ASA 2021 2a/B-NR)
- for patients with TIA and negative imaging, adding a follow-up MRI is reasonable (AHA/ASA 2021 2a/B-NR)
- follow-up CT/MRI is advisable before initiating anticoagulation to assess the extent of ischemia and exclude possible hemorrhagic transformation (best visualized on GRE/SWI) (AHA/ASA 2021 2b/B-NR)
Vascular imaging
- vascular imaging is used to detect stenosis or occlusion and assess probable etiology (atherosclerosis, dissection, FMD, etc.)
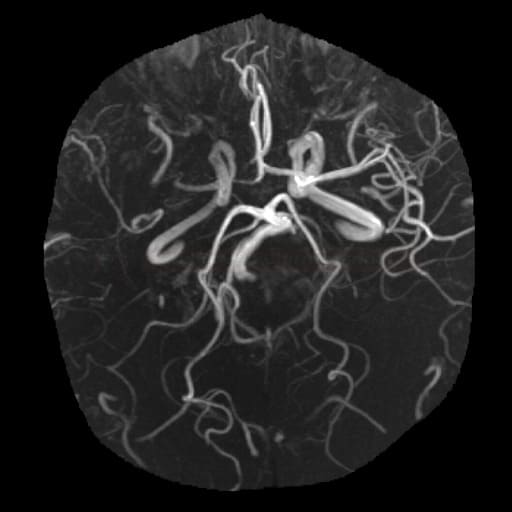
- in acute stroke patients, standard baseline imaging in most centers consists of brain CT+CT angiography (alternatively, MRI+MRA)
- in most medical centers, standard baseline imaging for acute stroke patients includes a brain CT+ CT angiography (alternatively MRI + MRA)
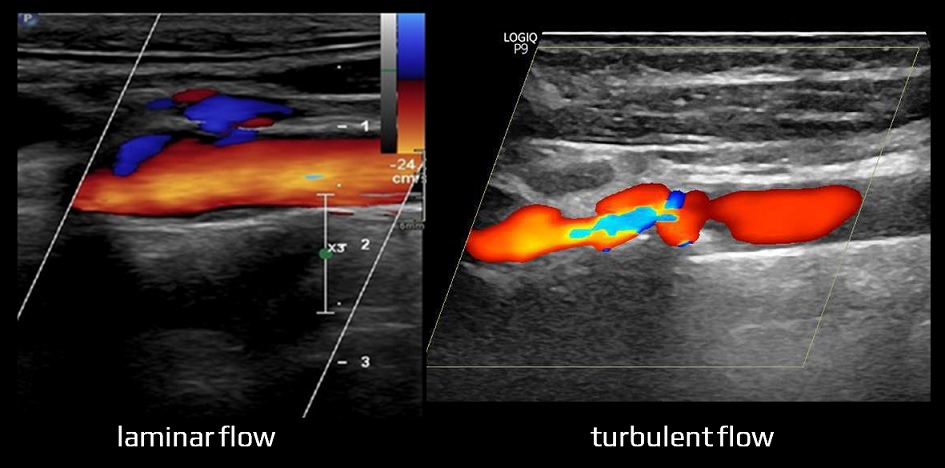
- patients with mRS 0-3, who are not eligible for recanalization therapy, should have vascular imaging within 24 h of admission (in the non-acute setting, neurosonology is the most commonly used method)
- both extra- and intracranial arteries should be evaluated (CTA from the aortic arch to the vertex is recommended)
- in acute stroke patients, standard baseline imaging in most centers consists of brain CT+CT angiography (alternatively, MRI+MRA)
- methods:
- CT angiography (see an in-depth tutorial on vascular assessment of stroke patients)
- MR angiography
- neurosonology
- in the acute stroke setting, transcranial Doppler with TIBI grading scale may be used to identify and monitor intracranial occlusion (the method has become less valuable with the availability of CTA and the advent of mechanical recanalization)
- DSA (most commonly used for endovascular procedures)
Arrhythmias detection
| Markers associated with a higher incidence of AFib |
| Electrophysiological |
|
| Biochemical |
|
| Morphological |
|
| Comorbidities |
|
Cardiac imaging
Laboratory tests
- assess vascular risk factors (AHA/ASA 2021 1/N-BR)
- consider testing for hypercoagulable states in selected cases
- screening is not advised due to the low detection rate and the high cost
- autoantibodies testing if vasculitis is suspected
- screening is not recommended due to the low detection rate and high cost
- cardiac enzyme testing (CK, CKMB, LD, high-sensitivity cardiac troponin)
- to exclude concomitant myocardial infarction (MI), which may be a source of cardioembolism
- troponin elevation is often attributed to a brain lesion rather than MI
- other tests
- toxicology (drugs)
- CSF analysis (vasculitis, DDx of neuroinfection)
- biopsy for conditions like vasculitis (brain and meninges), CADASIL (skin), etc.
- genetic testing (e.g., CARASIL, CADASIL, ACTA2, Grange syndrome, hypercoagulable states, and other genetic small vessel diseases
- consider screening for obstructive sleep apnea (OSA) (AHA/ASA 2021 2b/B-R)
Classification of ischemic stroke
- stroke is a highly heterogeneous disease in terms of etiology and clinical manifestation
- numerous classifications and subdivisions exist, many of them combining different elements (e.g., etiopathogenetic mechanism with risk factors or clinical presentation ), which can potentially lead to confusion
- there are many different ways to classify and categorize strokes
- some systems combine multiple factors, such as etiopathogenetic mechanism with risk factors or clinical presentation, which can potentially lead to confusion
- TOAST classification is widely recognized and seems to be most practical and useful for clinical purposes
Classification based on etiology and pathophysiology
|
Classification based on the appearance and location of ischemia (which may indicate etiology)
|
Classification based on duration (in conjunction with brain imaging)
|
Pathophysiologic classification
- the diagram provides a simplified representation of complex etiopathogenesis, where multiple mechanisms may coexist (e.g., arteriolopathy may have a thrombotic or atherothrombotic component, etc.)
Etiologic classification
- TOAST classification of stroke remains the most widely utilized system for categorizing stroke
- only a summary is presented below
- other improved classification systems include:
- the variability in the reported proportions of individual stroke subtypes is due to the age composition of the cohort and the diagnostic methods used).
- the proportional representation of stroke subtypes may change with the development of new diagnostic methods (e.g., long-term ECG monitoring increases the detection of paroxysmal AFib, thus decreasing the percentage of cryptogenic strokes, etc.)
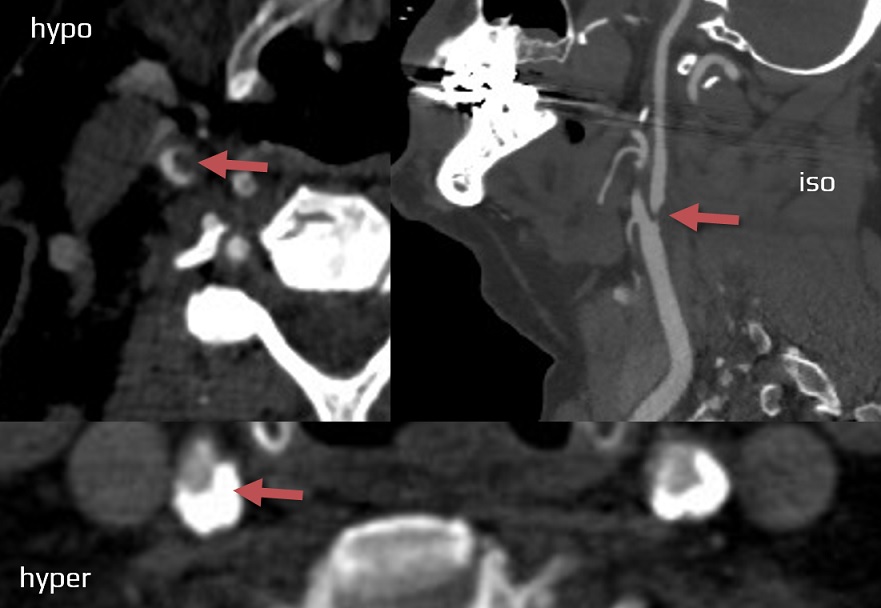
- significant stenosis (> 50%) or occlusion of a relevant extra- or intracranial artery due to atherosclerosis

- evidence of plaque hemorrhage, thrombus, plaque cap rupture, or angiogenesis indicates a high-risk plaque, irrespective of the degree of stenosis
- → assessement of atherosclerotic plaques
- search for aortic arch atherosclerosis, which is categorized as large artery atherosclerosis in both TOAST and CISS classifications
- brain imaging (CT/MRI)
- cortical lesion
- subcortical lesion > 1.5 cm (originally published)
- it is known, however, that even smaller lesions may be caused by branch artery atherosclerosis (see the CISS classification)
- cortical lesion
- common mechanisms of stroke in the TOAST 1 category:
- thromboembolism, atheroma embolization, or both (artery-to-artery embolization)
- the composition of the embolus may vary, ranging from a fragile fresh fibrin thrombus that easily fragments to a compact, tightly organized thrombus with solid plaque masses
- thrombosis or intraplaque bleeding leading to arterial occlusion
- occlusion of perforators caused by large plaques
- hypoperfusion due to severe stenosis (⇒ border zone infarcts)
- thromboembolism, atheroma embolization, or both (artery-to-artery embolization)
- cardioembolic stroke accounts for ~ 20-45% of all ischemic strokes (the proportion increases with advanced cardiac imaging and prolonged ECG monitoring)
- thromboembolism from the left atrium or ventricle is the most common; hypoperfusion is less frequent (⇒ typically resulting in border zone infarcts, as seen in conditions like cardiomyopathy)
- clinical syndromes and infarct features on brain imaging are usually indistinguishable from TOAST 1
- detection of left atrial thrombus on baseline CTA can help establish the correct etiological diagnosis
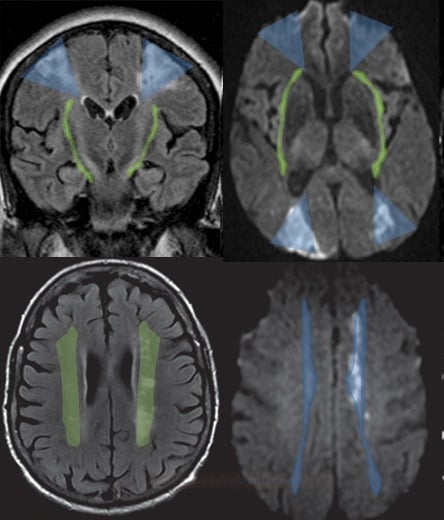
- arteriolopathy (primarily affecting arteries 0.4-0.5 mm in diameter) may lead to cerebral infarction or hemorrhages in deep brain structures
- the primary cause of arteriolopathy is lipohyalinosis
- particularly prevalent in patients with arterial hypertension
- lipohyalinosis is characterized by thickening of the arterial wall, which can result in vessel stenosis or occlusion
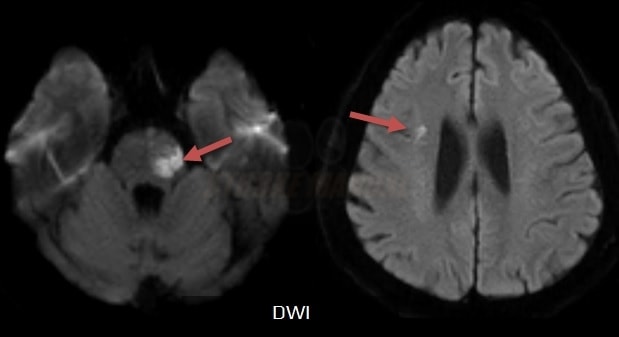
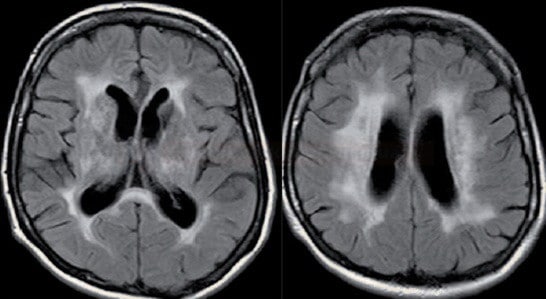
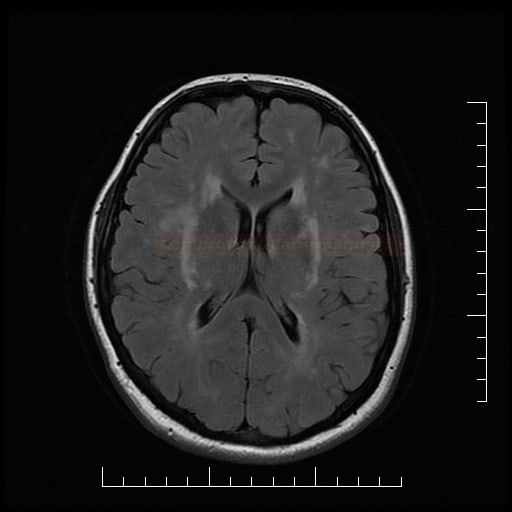
- brainstem or subcortical lacunar infarcts on CT/MRI (diameter < 1.5 cm)
 or subcortical ischemic leukoencephalopathy
or subcortical ischemic leukoencephalopathy 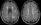 (→ FAZEKAS scale, ARWMC scale)
(→ FAZEKAS scale, ARWMC scale) - clinical presentation
- asymptomatic cases
- lacunar syndromes
- encephalopathy with cognitive impairment +/- pseudobulbar syndrome (attributable to the status lacunaris)
- + the presence of traditional vascular risk factors (hypertension, dyslipidemia, diabetes, etc.)
- distinguish non-arteriolopathic occlusion of perforating arteries
- atherosclerosis of the parent artery near the perforator origin – Branch Artery Disease (BAD) / Branch Occlusive Disease (BOD)
- infarcts tend to be larger compared to classic arteriolopathy and are more common in younger patients [Zhou, 2018]
- high-resolution MRI can be used for diagnosis [Petrone, 2016]
- embolization (originating from proximal arterial segments or cardioembolism)
- atherosclerosis of the parent artery near the perforator origin – Branch Artery Disease (BAD) / Branch Occlusive Disease (BOD)
- vasculitides
- non-inflammatory vasculopathies (dissection, FMD, etc.)
- genetic microangiopathies
- hematologic disorders
- iatrogenic insults, etc.
- cause of stroke remains undetermined due to insufficient diagnostic certainty
- ≥2 potential causes of stroke were identified (e.g., atrial fibrillation + carotid stenosis > 50%, significant carotid stenosis + microangiopathy, etc.)
- cryptogenic stroke (CS) – no etiology identified despite extensive evaluation [Bang, 2014]
- incomplete diagnostic evaluation