CEREBRAL HEMORRHAGE
Cerebral amyloid angiopathy (CAA)
Updated on 05/05/2024, published on 13/04/2023
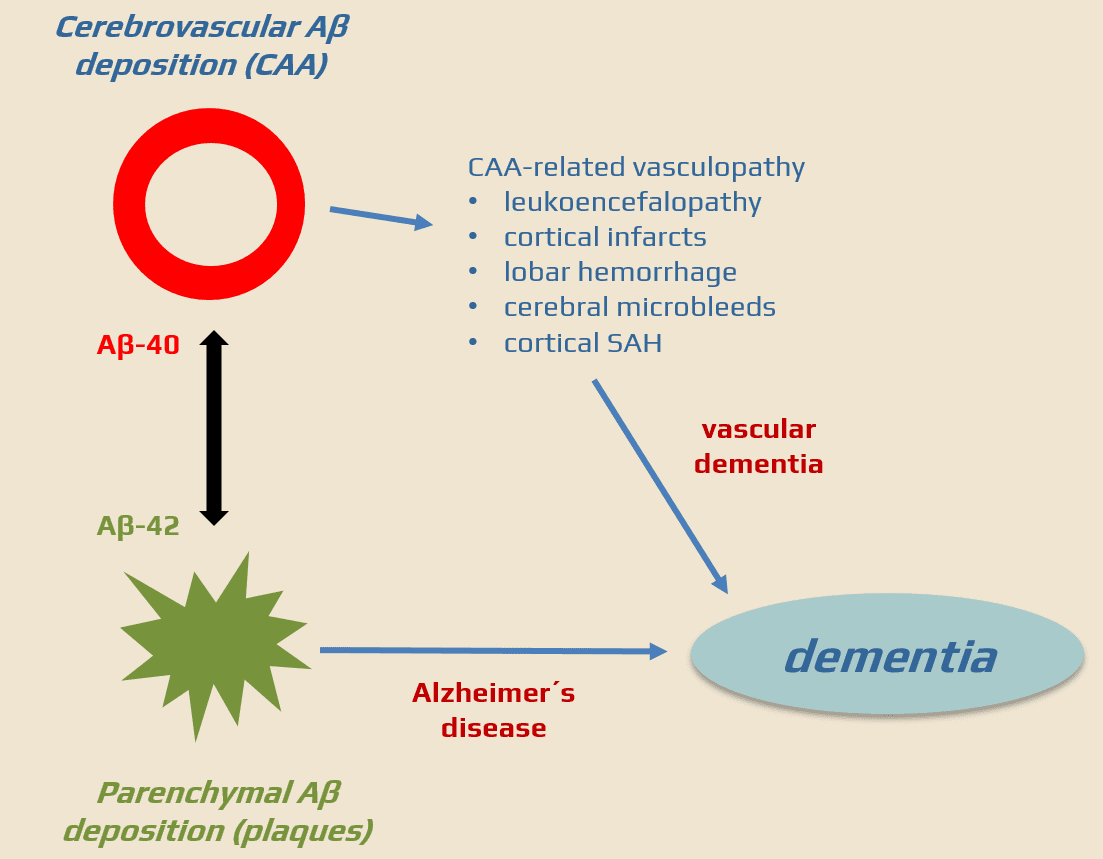
- Cerebral Amyloid Angiopathy (CAA) is a heterogeneous group of sporadic or familial disorders characterized by deposition of amyloid beta-peptide (Aβ) in the walls of small to medium-sized cerebral and leptomeningeal vessels in the absence of systemic amyloidosis; this accumulation can lead to vessel weakening, rupture, and cerebral hemorrhages
- sporadic form – affects mainly the elderly population
- Cerebral Amyloid Angiopathy-Related Inflammation (CAA-RI) is a relatively rare and aggressive variant of sporadic CAA with characteristic radiological findings
- rare familial forms (Icelandic, Arctic, or Dutch type) – occur at younger ages
- sporadic form – affects mainly the elderly population
- CAA is responsible for ~2-10% of primary intracranial hemorrhages (30-70% of lobar hematomas in elderly patients) [Chao, 2006]
- CAA may also present with transient neurologic symptoms or encephalopathy
The APP (Amyloid Precursor Protein) gene
- a transmembrane protein whose exact function is still under exploration; it is thought to be involved in neuronal development, synapse formation, and repair mechanisms within the nervous system
- mutations can lead to an altered production or structure of amyloid-beta, which may result in familial forms of Alzheimer’s Disease or CAA (e.g., the Dutch, Italian, Arctic, Iowa, Flemish, and Piedmont types)
- these mutations often result in increased production or altered clearance of Aβ peptides, which can accumulate in cerebral vessels
CST3 gene
- the CST3 gene encodes the protein cystatin C, a potent inhibitor of lysosomal proteases known as cathepsins
- cystatin C is critical for preventing the excessive breakdown of proteins by cathepsins in the body’s cells
- mutations in the CST3 gene are associated with Hereditary Cystatin C Amyloid Angiopathy (HCCAA), a type of familial CAA prevalent in Iceland
- genetic testing can help identify individuals at risk for HCCAA
- the CST3 gene and cystatin C have been studied for associations with other conditions such as Alzheimer’s disease, kidney disease, and cardiovascular risk
ITM2B gene
- the ITM2B gene encodes the Integral Membrane Protein 2B (aka BRI2)
- BRI2 is believed to play a role in protein processing and trafficking
- mutations are associated with familial forms of CAA, specifically the British and Danish types
- these mutations lead to abnormal processing of the BRI2 protein, resulting in the accumulation of amyloidogenic peptides in cerebral vessels
Pathology
- amyloid deposition in small to medium-sized cerebral arteries without systemic amyloidosis
- type 1 CAA- amyloid deposits in cortical capillaries, leptomeningeal and cortical arteries, and arterioles
- type 2 CAA – deposits are present in leptomeningeal and cortical arteries but not in capillaries
- there is some association with typical Alzheimer’s changes, such as neuritic plaques and neurofibrillary tangles
- the reason for the increased deposition of Aβ in sporadic CAA is still unclear (a combination of increased production of the peptide with abnormal clearance has been proposed)
- increased production is the dominant cause in familial forms
- apolipoprotein E (APOE) ε2 and ε4 are associated with an increased risk of CAA
Clinical presentation
- Transient Focal Neurological Episodes (TFNE), also called “amyloid spells“
- recurrent lobar intracerebral hemorrhage (ICH)
- convexal nontraumatic subarachnoid hemorrhage
- hemocephalus
- arteriolopathy with leukoaraiosis and encephalopathy
- development of vascular dementia in advanced stages of the disease
- Transient focal neurological episodes (TFNE), also called “amyloid spells”)
- positive symptoms – “aura-like” spreading paresis, visual phenomena (monocular blurred vision, flashes, teichopsia), limb twitching
- negative symptoms – transient focal symptoms – paresis, speech, and visual disturbances
- positive symptoms predominate
- typically, multiple stereotyped episodes occur, each lasting 10-30 minutes
- approx. 14% of CAA patients exhibit such symptoms [Charidimou, 2012]
- often caused by convexal SAH
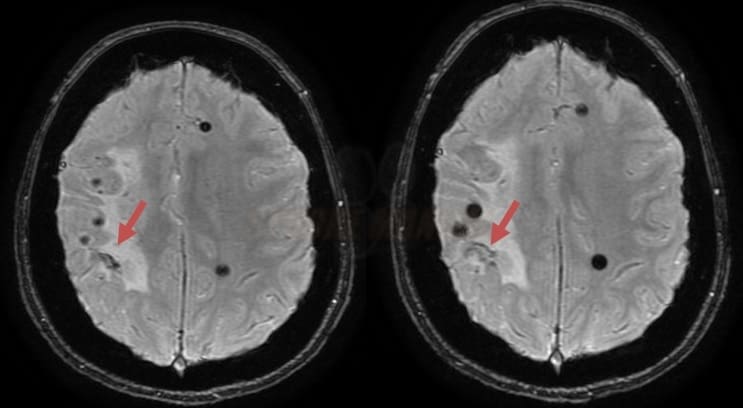
- FLAIR, DWI/ADC, and GRE/SWI are optimal for diagnostic workup
- frequently, convexial SAH is seen along with microbleeds or parenchymal hematoma, but also with recent lesions on DWI – etiology is heterogeneous
- FLAIR, DWI/ADC, and GRE/SWI are optimal for diagnostic workup
- spells may be confused with TIA (antithrombotic drugs further increase the risk of ICH) or stroke (high risk of ICH during thrombolysis)
- TFNE often precede subsequent ICH (37.5%/2 months)!! [Charidimou, 2012]
Diagnostic evaluation
- a definitive diagnosis can only be confirmed postmortem
- however, in elderly patients presenting with two or more lobar hemorrhages and concomitant microangiopathic changes on MRI, the diagnosis of CCA is highly probable
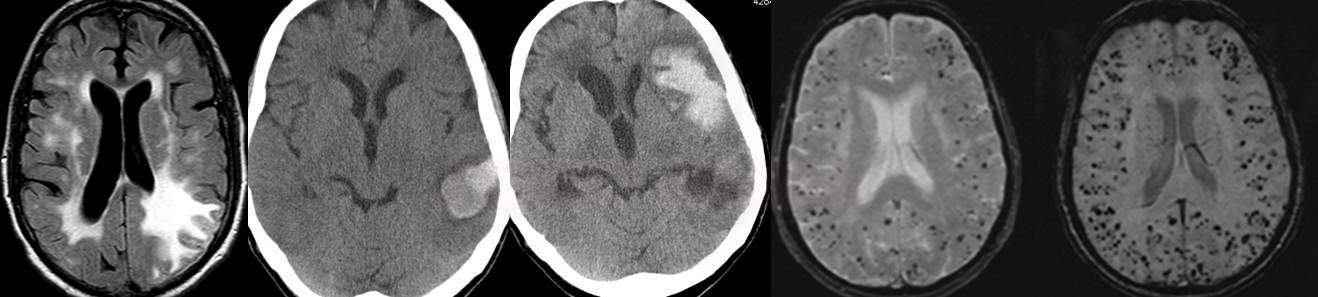
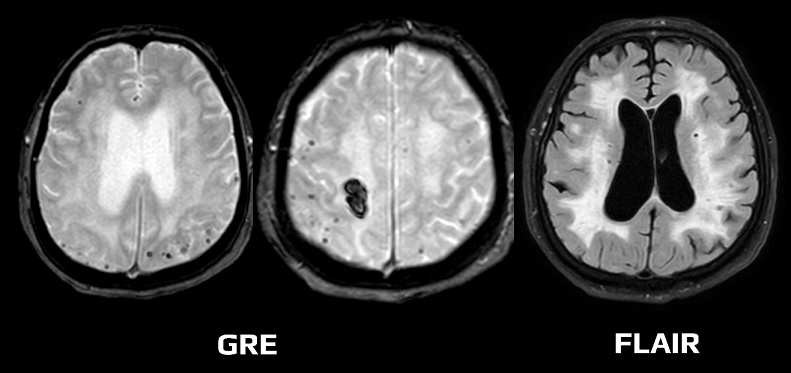
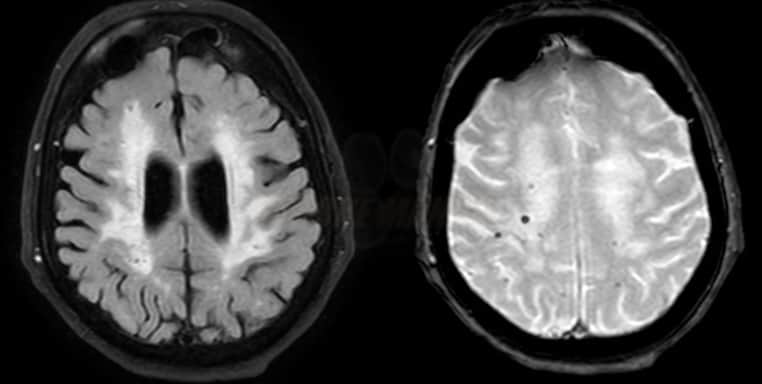
- typical findings on MRI include:
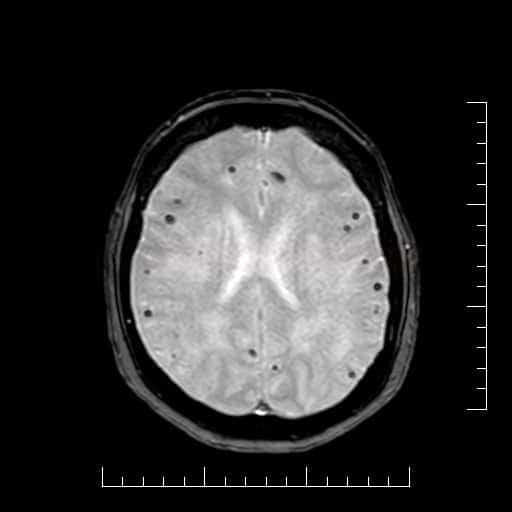
- lobar hemorrhages (often multiple)
- occurrence of finger-like projections (FLP) or SA extension are more likely in CAA
(Renard, 2012)
- occurrence of finger-like projections (FLP) or SA extension are more likely in CAA
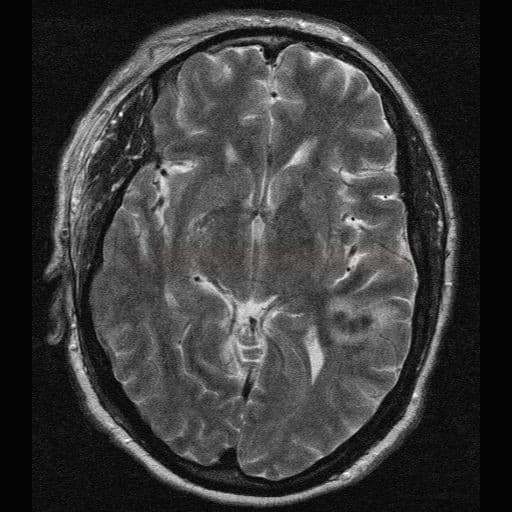
- superficial siderosis
- leukoencephalopathy (due to microangiopathy)
- if extensive, asymmetric vasogenic edema is present, think of CAA-related inflammation
- if extensive, asymmetric vasogenic edema is present, think of CAA-related inflammation
- microbleeds (best visualized on GRE/SWI)
- lobar hemorrhages (often multiple)
Diagnostic Boston criteria 2.0
The Boston criteria 2.0 were proposed in 2022 (Charidimou, 2022)
- possible CAA
- patients > 50 years of age, presenting with ICH, TFNE, or cognitive impairment
- MRI criteria:
- one strictly lobar hemorrhagic lesion on GRE/SWI OR at least one white matter feature (severe perivascular spaces or WML)
- absence of deep hemorrhagic lesions on GRE and other cause of hemorrhage (AVM, vasculitis, trauma, hemorrhagic trnasformation of ischemia, etc.)
- hemorrhagic lesion in the cerebellum is not counted as either lobar or deep hemorrhagic lesion
- one strictly lobar hemorrhagic lesion on GRE/SWI OR at least one white matter feature (severe perivascular spaces or WML)
- probable CAA
- MRI criteria:
- demonstrates either ≥ 2 of the following strictly lobar hemorrhagic lesions on MRI in any combination
- intracerebral hemorrhage (ICH), cerebral microbleeds (CMBs), cortical superficial siderosis (CSS) ) or convexity subarachnoid hemorrhage (convexity SAH)
- OR 1 lobar hemorrhagic lesion + white matter features:
- enlarged perivascular spaces in the centrum semiovale (>20 visible in one hemisphere)
- white matter hyperintensities in a multi-spot pattern (>10 subcortical FLAIR dots bilaterally)
- AND absence of deep hemorrhagic lesions and other causes of hemorrhage
- demonstrates either ≥ 2 of the following strictly lobar hemorrhagic lesions on MRI in any combination
- MRI criteria:
- probable CAA with supporting pathology
- clinical data + pathological tissue (from evacuated hematoma or biopsy) demonstrating some degree of CAA in the specimen
- definite CAA
- post-mortem examination demonstrating severe CAA on histology + signs of spontaneous lobal ICH and/or cortical cortical siderosis
Therapy
- there is no causal treatment for CAA
- monitor and treat arterial hypertension
- treat acute hematoma according to standard protocols → management of intracerebral hemorrhage
- antiplatelet and anticoagulant therapy increases the risk of bleeding ⇒ assess individual risk-benefit ratio in each patient (Biffi, 2010]
Anticoagulation in patients with suspected CAA
| Content available only for logged-in subscribers (registration will be available soon) |
Thrombolysis in patients with suspected CAA
- IVT is a reasonable treatment option for patients with CMBs < 10 (AHA/ASA 2019 IIa/B-NR)
- for patients with CMBs >10, IVT is associated with a higher risk of ICH; the expected benefit of treatment must outweigh the risk (AHA/ASA 2019 IIb/B-NR)
- consider IVT in a patient with severe deficit, taking into account premorbid health status and the presence of other risk factors