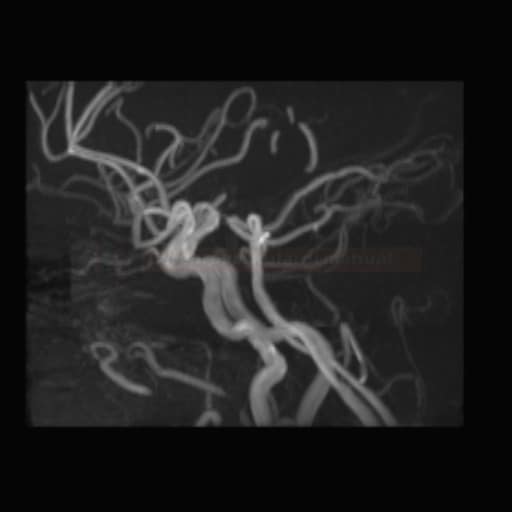
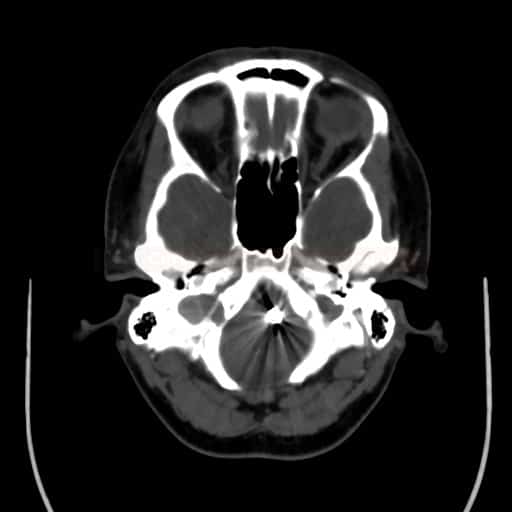
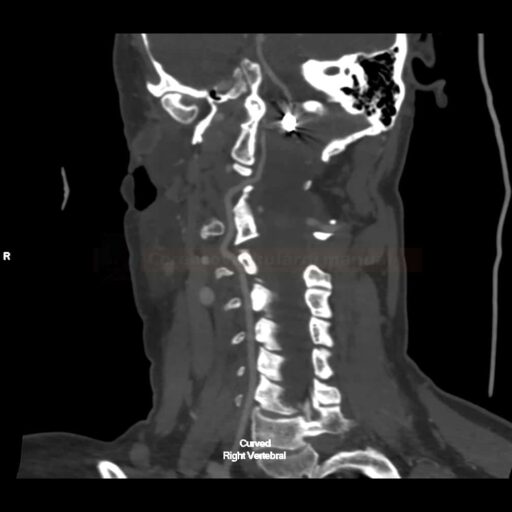
SUBARACHNOID HEMORRHAGE
Endovascular treatment of cerebral aneurysm
Updated on 12/12/2023, published on 03/04/2021
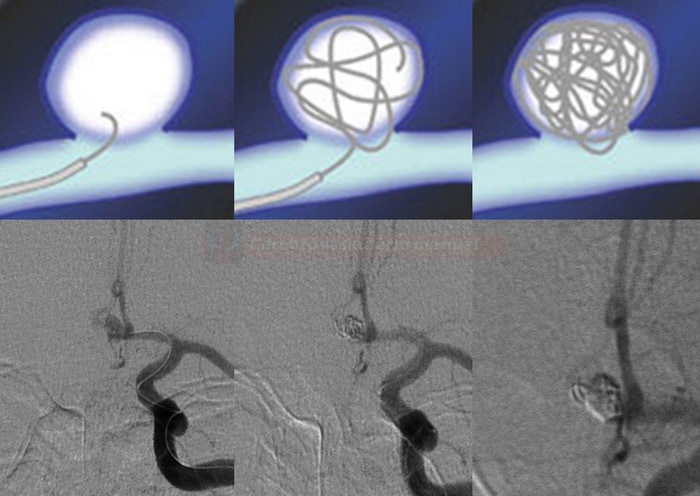
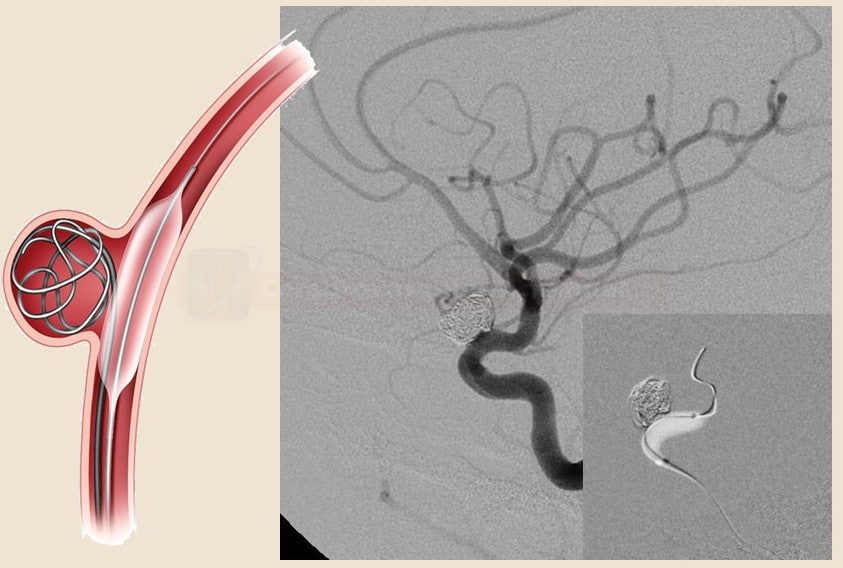
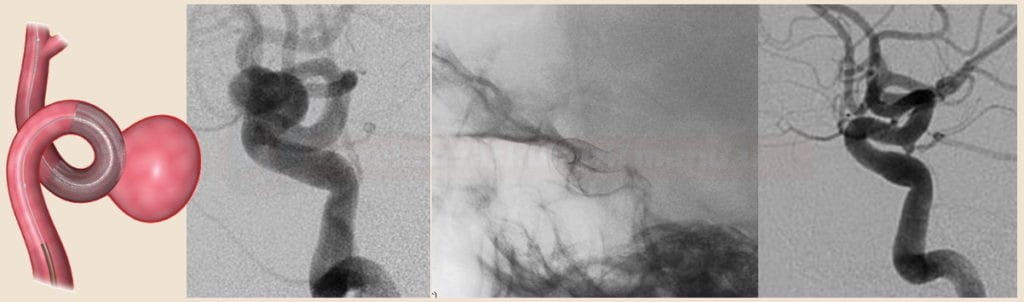
Coiling
- the most common procedure is coiling, in which the aneurysm sac is filled with detachable platinum coils → aneurysm is thrombosed and thus removed from circulation
- coils vary in stiffness, filament diameter, shape, size, length, and surface characteristics
- various 2D and 3D variants are available
- the coils are released from the guidewire using electrolytic, hydraulic, or mechanical methods
- coils vary in stiffness, filament diameter, shape, size, length, and surface characteristics
- the use of flow diverters, stent-assisted coiling, or balloon-assisted coiling expands the indications for coiling to include aneurysms with a wider neck (see below)

The endovascular procedure
- inform the patient about the planned procedure, its benefits, complications, and available alternatives
- antiplatelet drugs are necessary if stenting is anticipated
- place a Folley catheter and two intravenous cannulas before the procedure.
- coiling is usually performed under general anesthesia (GA) for several reasons:
- GA prevents dangerous movements during critical moments of the procedure, reducing the risk of arterial perforation)
- provides greater comfort for both the patient and the surgeon
- enables easier management of complications
- Transcranial Doppler (TCD) monitoring helps in detecting embolization from partially thrombosed aneurysms
- HEPARIN 3000-5000jj IV is initially administered to reduce the risk of thromboembolism
- for recent SAH, use only 2000 IU or administer heparin after the first coils have been applied to the sac
- monitor APTT during the procedure; adjust the heparin dose accordingly
- have PROTAMIN prepared in case of aneurysm rupture → see here
- access routes
- usually via the femoral artery
- alternatively, the radial, brachial, axillary, or rarely carotid artery may be used
- the guiding catheter and microcatheter must be continuously flushed with heparinized saline to prevent thrombus formation
- the stability of the first coil is crucial as it creates a structure that is filled with other smaller coils
- the decision to place the last coil is critical:
- incomplete filling of the aneurysm increases the risk of aneurysm progression and rebleeding
- conversely, a redundant coil is difficult to deploy and withdraw and may detach prematurely, potentially slipping into the parent artery
- after the procedure, admit the patient to the ICU for monitoring
Stent assisted coiling and other methods
- self-expandable stent with a 10% density (Boston, Leo, Cordis, Neuroform, Precise) is used to cover the neck; coils are subsequently inserted through the stent into the aneurysm sac
- the use of periprocedural heparin alone is adequate if the stent is used briefly during the procedure
- permanent stent placement requires prophylactic antiplatelet therapy (which should be avoided in acute SAH patients)
- start with ASPIRIN 100-300mg + CLOPIDOGREL 75mg 3 days before the procedure (> 24h after SAH onset)
- monitoring platelet inhibition through aggregometry is recommended to reduce the risk of thrombosis
- VerifyNow – target PRU (to measure P2Y12 receptor blockade) should be 60-200, target ARU (Aspirin Reaction Units) 350-549
- VerifyNow – target PRU (to measure P2Y12 receptor blockade) should be 60-200, target ARU (Aspirin Reaction Units) 350-549
- if platelet inhibition is not effective, consider a loading dose of ASA 300 mg+CLP 600mg
- alternatively, parenteral IIb/IIIa inhibitors may be administered during the procedure (immediately after stent and coil placement)
- after the procedure, continue with ASA 100-300mg+CLP 75 mg for 1-3 months
- specialized devices can be used to treat bifurcation aneurysms, such as PulseRider (Codman Neuro)
and pCONus (Phenox GmbH) are available
→ see here
- flow diverter´s density is ~ 30% (regular stents have ~ 10%)
- it diverts blood flow away from the aneurysm sac, promoting thrombosis within the aneurysm and reconstruction of the parent artery
- it is used in the treatment of intracranial aneurysms that are difficult to treat with conventional coiling techniques
- large and giant aneurysms in the supraophthalmic segments of the ICA, particularly those causing compression syndrome
- modeling of arteries with fusiform aneurysms where filling the cavity with spirals through the stent mesh would be difficult/impossible
- aneurysms < 2 mm or blister-like aneurysms
- recurrence of aneurysms previously treated with endovascular methods
- large and giant aneurysms in the supraophthalmic segments of the ICA, particularly those causing compression syndrome
- SILK, Pipeline, Pipeline Flex
[Wong, 2010]
- PIPELINE FLEX – initially approved for large and then for small and medium aneurysms (based on the PREMIER trial)
- treatment of wide-neck small/medium aneurysms resulted in high rates of complete occlusion without significant parent vessel stenosis and low rates of permanent neurological complications
- dual antiplatelet therapy (DAPT) (ASA+CLP) is given for 3 months post-procedure, followed by long-term ASA 81-325 mg (for at least 6 months)
- PIPELINE FLEX – initially approved for large and then for small and medium aneurysms (based on the PREMIER trial)
- for aneurysms > 15 mm, concurrent partial filling with coils is recommended to prevent wall rupture
- for large aneurysms, corticotherapy is recommended to prevent edema around the thrombosed aneurysm
- they consist of a metal mesh stent with an attached graft material that lines the inside of the artery
- when placed across the aneurysm, the stent graft immediately excludes the aneurysm from blood flow
-
disadvantages:
-
higher rigidity and difficulty passing through elongated arteries
-
high risk of thrombosis
-
- these devices are placed directly inside the aneurysm sac to promote its thrombosis while preserving blood flow in the parent artery
- this approach is useful for aneurysms with wide necks or challenging morphologies
- examples include
- Woven EndoBridge (WEB)
– WEB-IT trial
- Artisse (Medtronic)
- Medina (Medtronic)
- Contour Neurovascular System (Cerus Endovascular)
- Woven EndoBridge (WEB)
Complications of endovascular treatment
Coil/stent migration
- coil or stent migration is a rare (2-6% of cases) but potentially catastrophic complication that may lead to cerebral infarction
- it is more likely to occur in wide-necked aneurysms or after removal of an inappropriately placed coil
- several rescue devices have been introduced for the retrieval of displaced material
- stent-retrievers [ 2015]
- MERCI catheter [Kung, 2012]
- sometimes, open surgery may be necessary to remove the displaced coil [Turek, 2015]
Periprocedural aneurysm rupture
- mostly, recently bleeding aneurysms are affected
- prevention: using general anesthesia (GA) to prevent patient’s movements, use of appropriate microcatheters and spirals
- in case of rupture, counteract heparinisation with protamine sulfate (→ see here) and try to obliterate the aneurysm as soon as possible
- if the aneurysm cannot be secured endovascularly, consider urgent neurosurgical intervention
- occasionally, bleeding may not become apparent until several days after the arterial wall is injured
Thromboembolic complications
- these complications mostly occur early; late thromboembolism is quite rare
- prevention strategies include:
- adequate heparinization
- gentle catheter manipulation
- continuous flushing of the guiding catheter and microcatheter
- treatment of the major artery thrombotic occlusion:
- whenever possible, thrombectomy with a stent-retriever is preferred approach [Briganti, 2016]
- Ilb/IIIa platelet receptor blockers can be given intravenously or as a small intra-arterial bolus [Jones, 2008]
- local tissue plasminogen activator (tPA) usage carries an increased risk of bleeding
(Integrilin) usually 1mL/2mg or 1mL/0.75 mg
- IV bolus: 0.2 mg/kg within 5 minutes, followed by infusion (Sedat, 2014)
- IV infusion: 5mL(10mg) + 45mL of NS (1mL=0.2 mg) …… 3mL/h (0.6 mg/h) 0.125ug/kg/min (max 10ug/min!)
- IA bolus: 5mL /10mg) + 45 mL of NS (1mL=0.2 mg) …… bolus 10 mL (2mg) every 5 minutes till max dose 10 mg
(Reopro) 1mL/2mg
- a monoclonal anti-glycoprotein IIb/IIIa receptor antibody
- IV bolus: 0.25 mg/kg within 5 minutes, followed by infusion
- IV infusion: 5mL (10mg) + 45mL NS (1mL of solution = 0.2 mg) …… 3mL/h (0.6 mg/h) 0.125ug/kg/min (max rate 10ug/min!)
- IA bolus: 5mL (10mg) + 45 mL NS (1mL of solution = 0.2 mg) …… bolus 10 mL (2mg) every 5 minutes, max dose 10mg
Vasospasms
- some vasospasms are transient and may resolve after catheter removal or displacement
- continuous IV nimodipine (if available) may be started from the beginning of the procedure
- if vasospasm persists, consider the use of local vasodilators
(Dilceren / Nimotop) usually 1 mL/0.2 mg
- IV: 1-2 mg/h (5-10mL/h) by continuous infusion + 10 ml/h NS concomitantly
- AEs: hypotension, tachycardia
- combine with vasopressors in case of hypotension
- parenteral administration is not more effective than oral administration in preventing vasospasm
- infusion pumps with polyethylene tubing and needles with polyethylene handles (or all-metal) must be used, infusion solution is light sensitive, must not be used in direct sunlight
- IA: 50 mL (10mg) + 50mL NS (to get 10% solution; 10mL containing 1mg) … IA infusion 1mL/min (0.1mg /min)
-
0.5-2 mg into a single artery, total max dose 5 mg [Kim,2009]
-
(Verapamil / Isoptin / Lekoptin) usually 1mL/2.5 mg
- IA: bolus 1-3 mg (or 44 μg/kg) locally [Feng,2002]
- AEs: hypotension, bradycardia
(Perlinganit)
- IA: 0.5-2 mg as a slow bolus
(Asicord / Primacor)
- IA: 1mg/ml – 0.25 mg/min – infused for 30 minutes
- regression of vasospasm with combined IA and subsequent IV administration has been reported [Arakawa, 2001] [Fraticelli, 2008]
- IV administration alone seems to be effective [Crespy, 2019]
(Magnesium sulfate) usually 1mL/40 mg or 1mL/80mg
- IA: 10mL (1g) + 28 mL NS …. 0.25-1 g per artery [Shah, 2009]
- may be used in combination with nicardipine (2.5-20.0 mg/h for 30-60 min)
- magnesium is believed to inhibit cerebral vasospasm by causing smooth muscle relaxation and vasodilation by mechanisms similar to the calcium channel antagonists
- IV: a large phase III study showed no significant benefit in cerebral vasospasm prevention or improved favorable outcomes when administered via IV infusion (Wong, 2010)
(Papaverine hydrochloride) 1mL/30mg
- IA: 300mg (10 mL) + 100mL of NS (solution concentration is < 3mg/mL) …. IA infusion 3mL/min for 30 minutes or 1.3 mL/min for 60 minutes [Kassel, 1992][Clouston, 1995]
- usually not used due to AEs
Postprocedural medication
| Content available only for logged-in subscribers (registration will be available soon) |
Follow-up of coiled aneurysms
- compared to clipping, coiling requires a prolonged time before the aneurysm is entirely excluded from the circulation
- there is a higher percentage of incomplete closure with partial sac filling, necessitating re-embolization (⇒ perform MRA/DSA checks for early detection of recanalization or aneurysm overgrowth)
- in the ISAT study, the need for a repeat procedure in the endovascular arm was 17.4% versus 3.8% in the surgery arm
- CTA is not appropriate for follow-up because of significant coil-induced artifacts
- completely filled aneurysms that remain stable at six months can be considered cured, requiring no further follow-up
- patients with incompletely embolized aneurysms must be closely monitored; sometimes, repeat embolization is necessary
- BUT: the outcome in terms of disability-free survival at 1 year is significantly better with endovascular coiling
- follow-up X-ray of the skull is recommended 3-6 weeks after the procedure
- check the position of the coils in the baseline projections (whether the shape and structure of the spiral cluster changes and whether their position in relation to the skeletal structures changes)
- changes in shape or position raise suspicion of compression of the spirals in the sac or their insertion into a thrombus (⇒ perform MRA/DSA)
- contrast-enhanced MRA – 3-6 months after the procedure (not earlier because of the risk of coil dislocation in the magnetic field)
- in most cases, ce-MRA can replace DSA
- if residual filling of the aneurysm is suspected, add DSA
- DSA – remains the most reliable method to assess embolization stability, providing accurate comparison and information about the hemodynamics of residual pouch filling
Results of endovascular therapy
- the technical success of endovascular treatment is influenced by several factors:
- the experience of the interventional radiologist
- the patient’s age and clinical condition
- the presence of vasospasm or elongation and kinking of extracranial arteries
- the experience of the interventional radiologist
- disadvantages include less stable closure in large aneurysms, wide-neck aneurysms, or cases of intrasaccular thrombosis
- in small aneurysms, complete occlusion is achieved in ~70-90% of cases
- in large to giant aneurysms, the rate is ~ 50%
- in small aneurysms, complete occlusion is achieved in ~70-90% of cases
- incomplete occlusion results in residual sac filling
- causes of incomplete occlusion:
- incomplete filling of the neck by coils
- compression of the coils before the neck is covered with neointima
- insertion and displacement of the coils into the thrombus that was present inside the aneurysm during the procedure
- incomplete filling of the neck by coils
- incomplete occlusion is a risk factor for aneurysm expansion; endovascular treatment may be repeated, and additional coils added to the sac


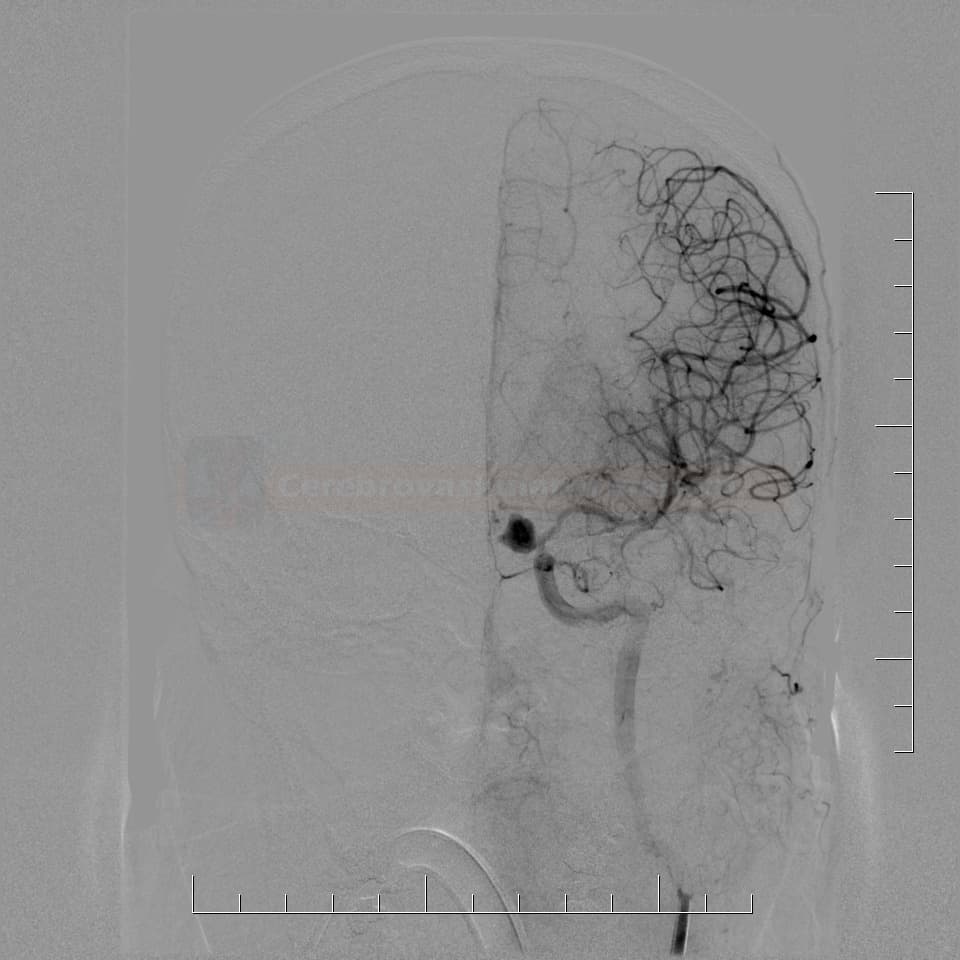
![Extraction of migrated coil using MERCI catheter [Kung, 2012] Extraction of migrated coil using MERCI catheter [Kung, 2012]](https://www.stroke-manual.com/wp-content/uploads/2021/04/coiling_complication-01.jpg)
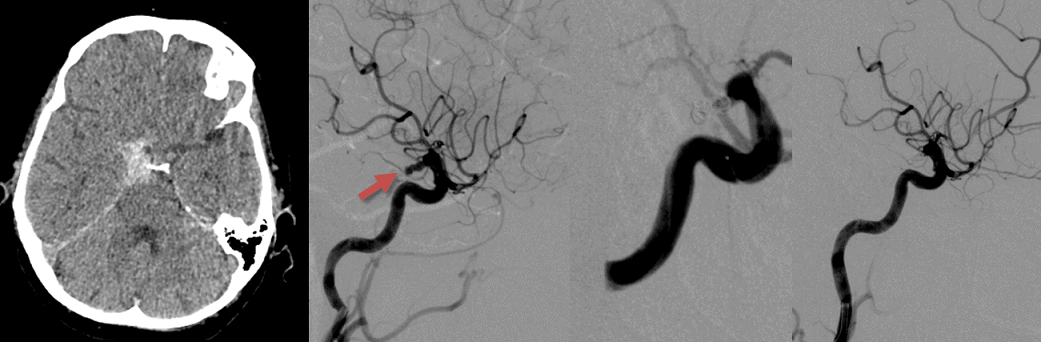
![AICA aneurysm rupture (black arrow) with blood extravasation (red arrows) [Sharma, 2011] AICA aneurysm rupture (black arrow) with blood extravasation (red arrows) [Sharma, 2011]](https://www.stroke-manual.com/wp-content/uploads/2021/04/coiling_complication-aneurysm-rupture.jpg)
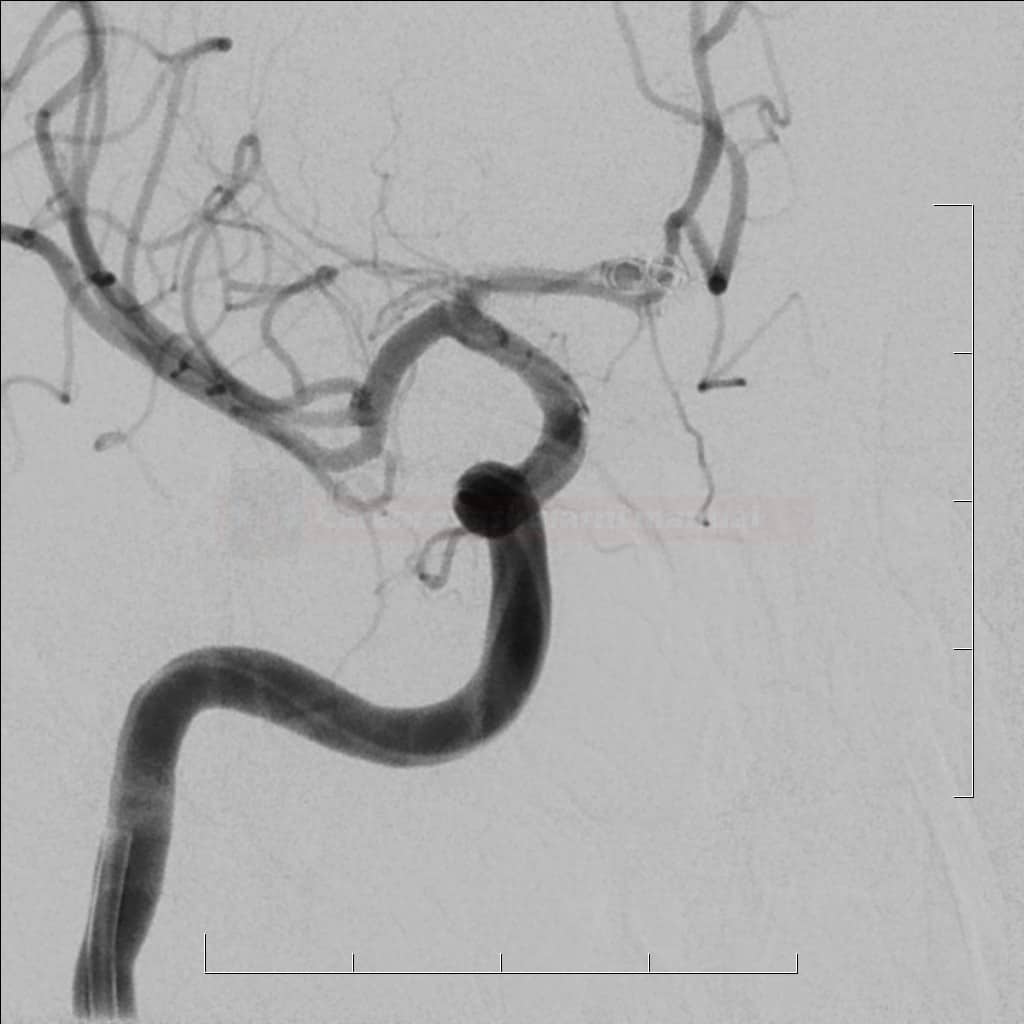
![Thrombotic complication during coiling of an aneurysm at the top of the basilar artery. Recanalization was achieved with IA application of abciximab (0.25mg/kg) [Jones, 2008] Thrombotic complication during coiling of an aneurysm at the top of the basilar artery. Recanalization was achieved with IA application of abciximab (0.25mg/kg) [Jones, 2008]](https://www.stroke-manual.com/wp-content/uploads/2021/04/coiling-complications-thrombembolic.jpg)