MEDICATION / ANTICOAGULANT THERAPY
Reduced-dose DOACs
Updated on 14/08/2023, published on 04/10/2022
- dosing of direct oral anticoagulants (DOACs) can be classified into:
- standard (e.g., 20 mg of rivaroxaban, 150 mg of dabigatran, etc.)
- reduced (e.g., 15 mg of rivaroxaban, 110 mg of dabigatran, etc.)
- low-dose (e.g., rivaroxaban 2x 2.5 mg or 1x 10 mg daily)
- in stroke prevention (typically Afib patients), use the standard dose whenever possible
- reduce dose in selected patients only (Lee, 2020)
- in general, the reduced dose of DOACs is non-inferior to warfarin when correctly indicated [Wang, 2019]
- off-label dose reduction may be harmful due to excessive thromboembolic risk
- the low-dose DOACs are currently used:
- for extended VTE thromboprophylaxis unless the risk of recurrent VTE is high (Cohen, 2019)
- for stable PAD/CAD patients – see COMPASS trial criteria (rivaroxaban 2.5 mg twice daily + ASA)
Pharmacokinetics of DOACs
- DOACs (dabigatran, apixaban, rivaroxaban, edoxaban) have a linear relationship between dose, plasma concentration and anticoagulant effect
- lower doses ( 110 mg dabigatran, 15 mg rivaroxaban, 2.5 mg apixaban and 30 mg edoxaban) lead to lower plasma drug levels
- dabigatran at 150 mg is associated with a 40% increase in mean plasma drug concentration
- apixaban dose reduction is associated with a 50% decrease in maximum plasma drug concentration
- a reduction in the dose of edoxaban leads to a decrease in plasma levels and anti-Xa activity of approximately 50%
- if at least 1 of the following factors is present (weight ≤ 60 kg, GF 30-50 ml/min, concomitant treatment with a potent glycoprotein P inhibitor (e.g., verapamil)), 50% dose reduction results in only a 25% decrease in plasma concentration
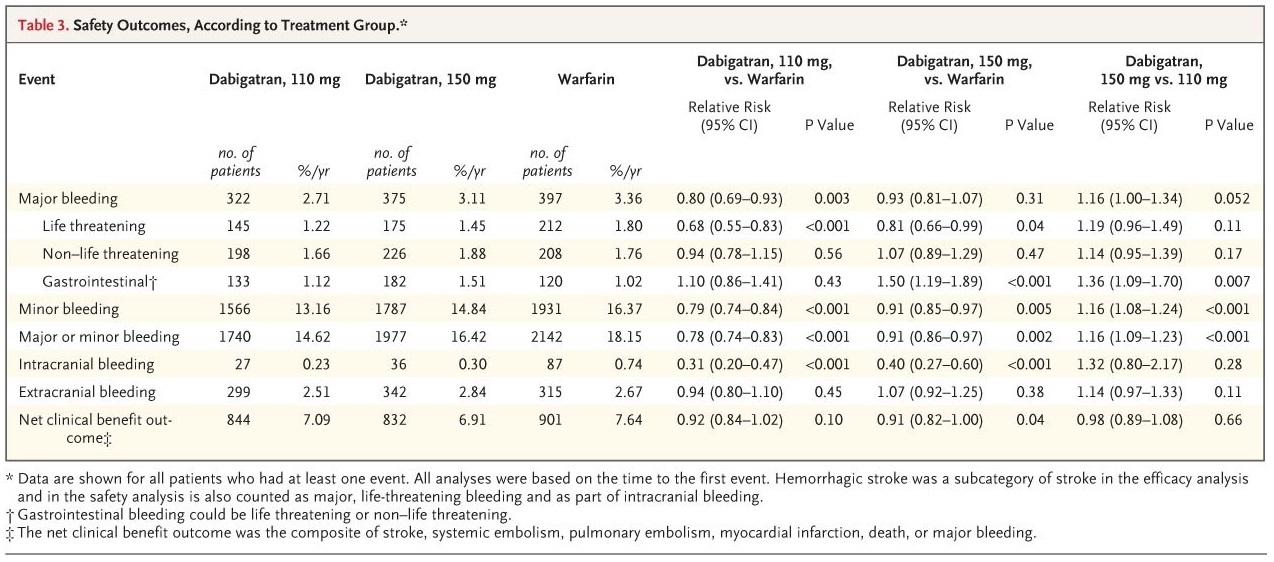
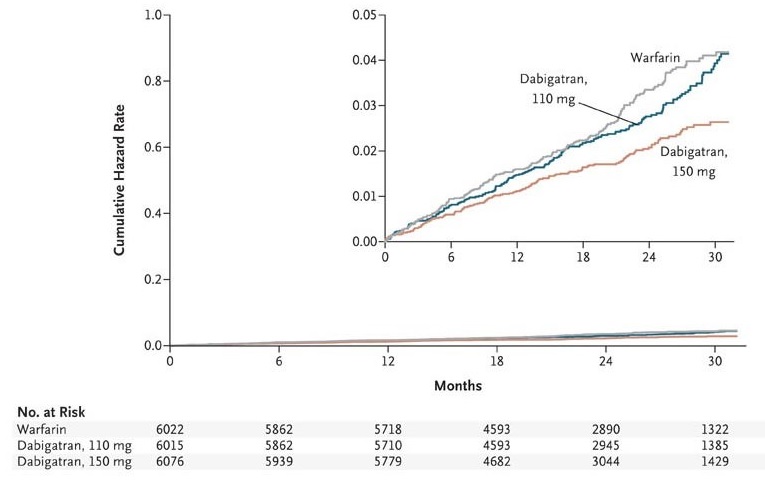
Dabigatran
- in the RE-LY trial, patients were randomized to receive either 150 or 110 mg of dabigatran
- plasma dabigatran concentrations were higher at the 150 mg dose than at the 110 mg dose
- efficacy was higher at the 150 mg dose, and safety was lower than at the 110 mg dose (thus, the goal of the lower dose was to reduce the anticoagulant effect)
- dabigatran at 150 mg showed higher efficacy and comparable safety to warfarin
- dabigatran at 110 mg showed efficacy comparable to warfarin with increased safety
Xabans
- in the ROCKET AF, ARISTOTLE, and ENGAGE AF-TIMI 48 trials, two different predefined groups of patients received higher or lower doses of rivaroxaban, apixaban, or edoxaban depending on whether they had factors that increased plasma drug concentrations (age ≥ 80 years, creatinine clearance 30-49 mL/min, etc.)
- dose reduction aimed at maintaining appropriate anticoagulant intensity despite these factors
Dosing of DOACs
| CrCl | ≥ 50 mL/min (0.83 mL/s) |
30-49 mL/min (0.5-0.82 mL/s) |
15-29 mL/min (0.25-0.49 mL/s) |
< 15mL/min (0.25 mL/s) |
| dabigatran (PRADAXA) |
2x 150 mg | 2x 110mg | ||
|
2x 110mg |
||||
| apixaban (ELIQUIS) |
2x 5 mg | 2x 2.5 mg | ||
| 2x 2.5mg if ≥ 2 of risk factors are present: age ≥ 80 let, creatinine > 133 umol/L, weight ≤ 60 kg |
||||
| rivaroxaban (XARELTO) |
1x 20 mg (with food) | 1x 15mg (with food) | ||
| edoxaban (LIXIANA) |
1x 60 mg | 1x 30mg | ||
|
1x 30mg |
||||
- off-label dose reduction of DOACs is associated with a higher risk of thromboembolism [Lee, 2020]
- dose reduction to increase the safety of anticoagulant therapy is acceptable only with dabigatran
- use reduced doses with caution – mild-moderate renal insufficiency is not automatically a reason to reduce the dose of dabigatran (it may not lead to a presumed significant reduction in the risk of bleeding but may increase the risk of thromboembolism [Eikelboom, 2011]
- with rivaroxaban, apixaban, and edoxaban, dose reduction should be done only in the presence of factors increasing the plasma concentrations to avoid the excessive anticoagulant effect
- dose reduction in the absence of these factors is an off-label approach and is associated with uncertain, probably reduced efficacy of the drug
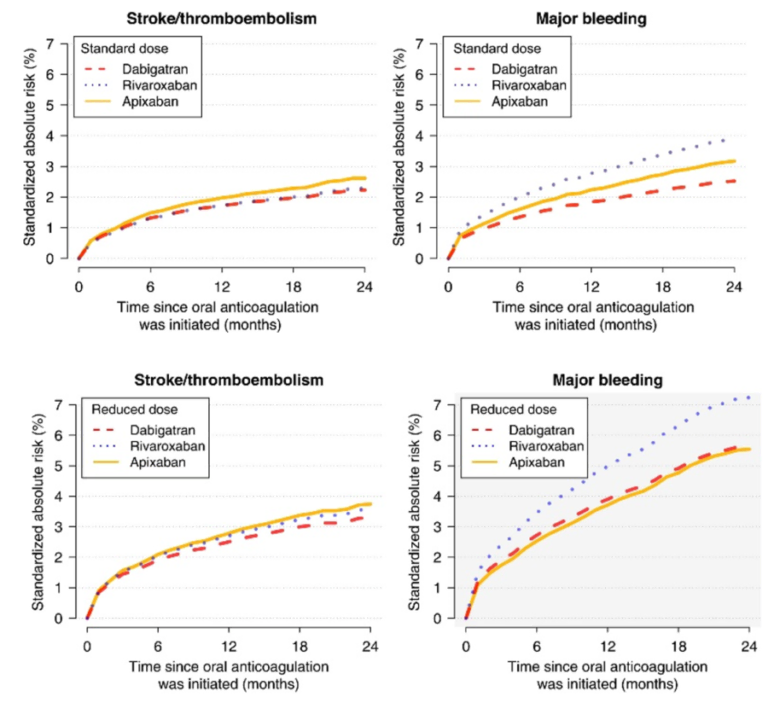
Comparison of the effectiveness of reduced doses
- according to a large meta-analysis, all dose-reduced DOACs (if properly indicated) are non-inferior to warfarin in patients with AF (in particular Asians) [Wang, 2019]
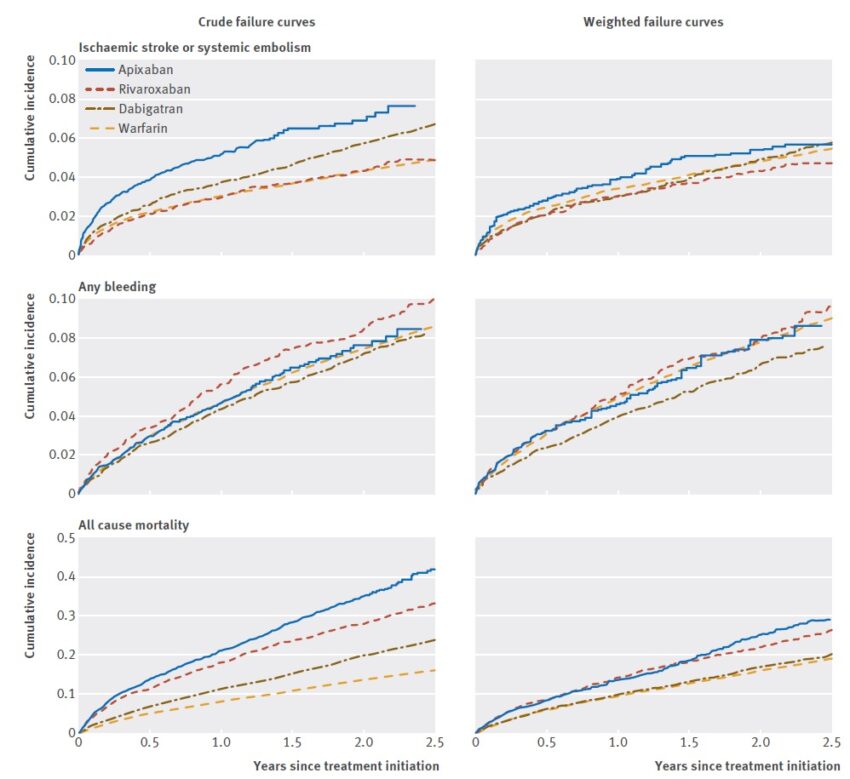
- according to the Danish registry [Nielsen, 2017]
- apixaban at a dose of 2x 2.5 mg is associated with a trend toward higher rates of thromboembolism compared to warfarin
- rivaroxaban 1x 15 mg and dabigatran 2x 110 mg had a trend towards a lower incidence of thromboembolism compared to warfarin
- rates of bleeding (the principal safety outcome) were significantly lower for dabigatran but not significantly different for apixaban and rivaroxaban compared with warfarin
- according to another analysis of Danish registries, standard and reduced doses showed no significant risk difference for thromboembolism. Rivaroxaban was associated with a higher risk of major bleeding compared to apixaban and dabigatran, and dabigatran was associated with lower intracranial bleeding risk compared with rivaroxaban and apixaban. [Staerk, 2018]