ADD-ONS / MEDICATION / ANTICOAGULANTS
Edoxaban (LIXIANA)
Updated on 24/01/2024, published on 25/10/2022
Edoxaban (LIXIANA) is an oral anticoagulant drug used to treat and prevent VTE and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. It is used as an alternative to warfarin, which does not require laboratory monitoring or dietary restrictions. Edoxaban belongs to a group of drugs called direct oral anticoagulants (DOACs)
Indications
- Primary and secondary stroke prevention in non-valvular atrial fibrillation (NVAF) – ENGAGE AF-TIMI 48 trial
- Treatment and prevention of venous thromboembolism (VTE) – deep vein thrombosis (DVT) and pulmonary embolism (PE) – Hokusai-VTE
- edoxaban has not yet received approval for postoperative prophylaxis for venous thromboembolism (VTE)
Contraindications
- hypersensitivity to edoxaban (or any component of the formula)
- pregnancy/breastfeeding (generally, DOACs are not recommended)
- edoxaban should be used with caution only if the benefits outweigh the risks as there haven’t been enough studies during pregnancy
- the drug passes into breast milk
- renal functions → use the Cockcroft-Gault formula!
- all xabans are contraindicated with CrCl <15 mL/min (0.25 mL/s)
- edoxaban should also not be used in patients with CrCL > 95 mL/min (Poulakos, 2017)
- the ENGAGE AFTIMI 48 study demonstrated that subjects with nonvalvular atrial fibrillation patients and CrCL higher than 95 mL/min had a more significant risk of ischemic stroke with a 60 mg daily dosage of edoxaban compared to subjects managed with warfarin
- active bleeding
- congenital or acquired lesions/conditions considered a significant risk factor for major bleeding:
- active or recent GI ulcerations, presence of malignant tumors
- a recent brain or spinal injury or surgical procedure
- eye surgery, vascular retinopathy
- recent intracranial hemorrhage
- the known presence or suspected esophageal varices, AVMs, vascular aneurysms, or other vascular anomalies (relative contraindication)
- bronchiectasis or history of pulmonary bleeding
- uncontrolled severe arterial hypertension
- spontaneous/pharmacological (other anticoagulants) disorders of hemostasis
- liver disease
- all DOACs are contraindicated in patients with liver disease associated with coagulopathy and clinically apparent risk of bleeding
- avoid edoxaban in patients with Child-Pugh C or patients with coagulopathy related to liver disease
- no dosage adjustment is necessary for patients with mild hepatic impairment
- concomitant therapy (especially potent P-glycoprotein inhibitors)
- ketoconazole, itraconazole
- macrolides
- conditions requiring VKAs
- avoid DOACs in patients with a mechanical valve or moderate-severe mitral stenosis (contraindication)
- not recommended in patients with prosthetic heart valves or rheumatic heart disease
- antiphospholipid syndrome (APS)
- avoid DOACs in patients with a mechanical valve or moderate-severe mitral stenosis (contraindication)
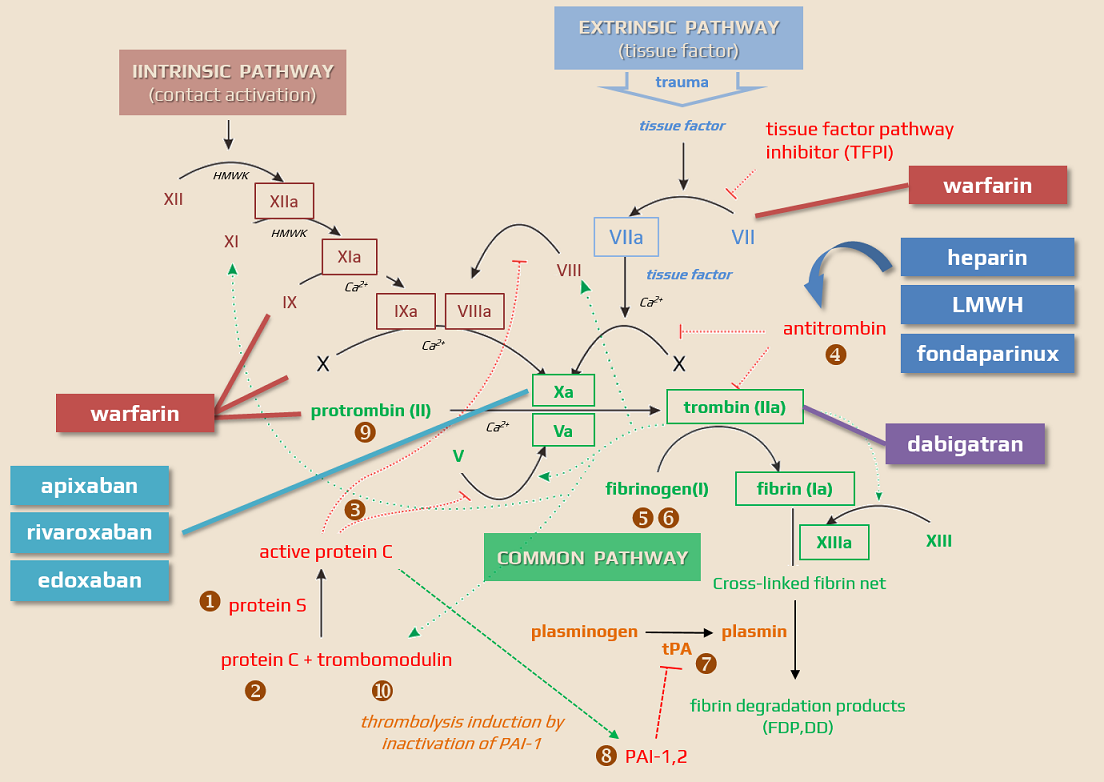
Mechanism of action
- direct, highly selective reversible inhibitor of activated factor Xa (fXa)
- pharmacodynamic effects are predictable and correlate with the dose and concentration of edoxaban
- edoxaban inhibits both free and clot-bound factor Xa
- edoxaban exerts its effects on the intrinsic and extrinsic pathway of the coagulation cascade, prolonging clotting tests of both prothrombin time (PT) and activated partial thromboplastin time (aPTT)
| Direct anticoagulants They inactivate the clotting factors present in the plasma |
Indirect anticoagulants They affect clotting factors by reducing their liver production |
|
| Direct thrombin/factor Xa inhibitors These drugs bind to thrombin/factor Xa and thereby block their function |
Indirect thrombin/factor Xa inhibitors These drugs activate antithrombin |
|
|
||
|
||
|
||
Pharmacokinetics and pharmacodynamics
- active drug with rapid absorption and onset of action
- peak plasma concentration reached within 1-2 h
- half-life: 10-14h
- can be administered both fasting or after the meal
- do not administer edoxaban to patients with CrCl < 0.25ml/s (15 ml/min)
- elimination by the liver (50%) and kidney (50%); there is only minimal degradation via CYP3A4
- dose reduction for weight < 60 kg is recommended
Interactions
- no significant interaction with atorvastatin, omeprazole, digoxin, or midazolam
- patients at risk:
- with renal insufficiency
- with hepatopathy-induced coagulopathy ⇒ avoid DOACs!
| Content available only for logged-in subscribers (registration will be available soon) |
Administration and dosing
- used orally with or without food once a day
| edoxaban (LIXIANA) | stroke prevention |
VTE prevention and therapy (LMHW bridging for 5-10 days) |
VTE prevention in the hip or knee surgery (THR, TKR) |
| CrCl > 0.83 mL/s (>50 mL/min) with no risk factors (low weight, concomitant drugs) |
1x 60 mg |
1x 60 mg |
– |
| CrCl 0.25-0.83 mL/s (15-50 mL/min) or some of the risk factors: P-gp inhibitors (cyclosporine, dronedarone, erythromycin, ketoconazole) weight ≤ 60 kg |
1x 30 mg |
1x 30 mg (after 5 days of LMHW bridging) |
– |
with CrCl > 95 mL/minute, replace edoxaban with another DOAC (FDA 2015)!
Monitoring
- clinical monitoring usual for all anticoagulated patients
- specific laboratory monitoring is helpful in selected cases
Complications, adverse events
Other adverse events
Overdose
| Content available only for logged-in subscribers (registration will be available soon) |