ADD-ONS / MEDICATION / ANTICOAGULANTS
Apixaban (Eliquis)
Updated on 22/04/2024, published on 20/09/2023
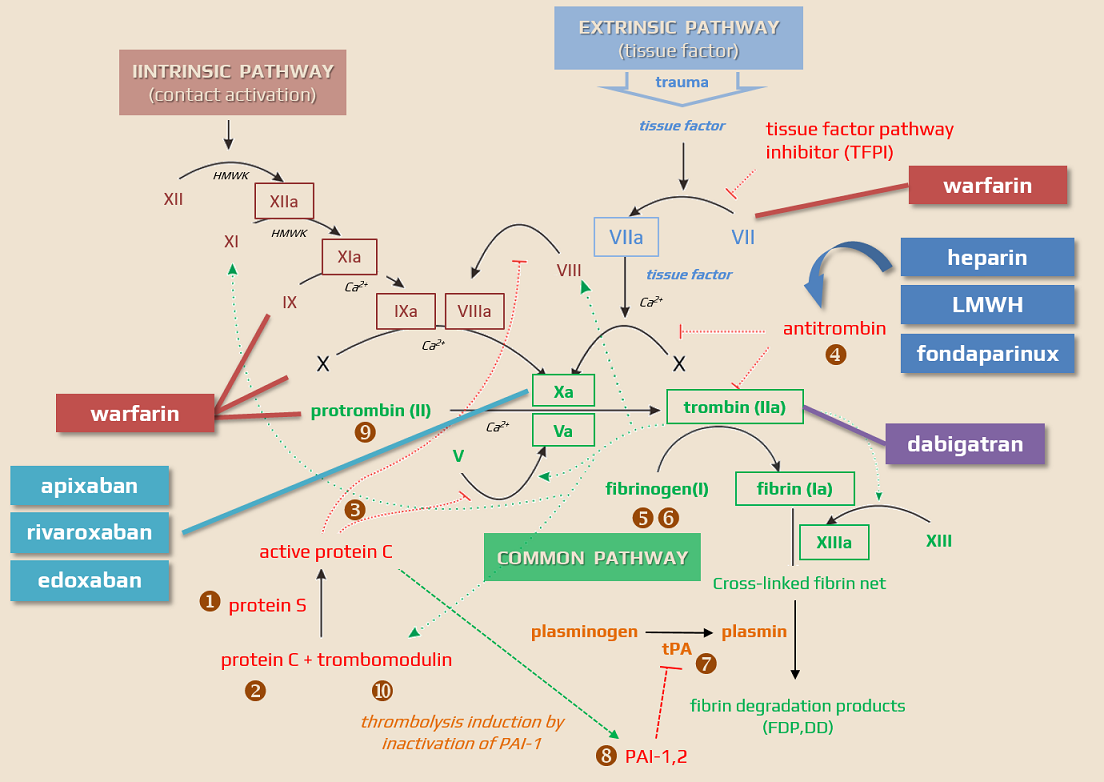
Apixaban (ELIQUIS) belongs to a group of drugs called direct oral anticoagulants (DOACs). It is a direct Factor Xa inhibitor and is used as an alternative to warfarin for the treatment and prevention of VTE and stroke in people with nonvalvular atrial fibrillation (NVAF). Unlike warfarin, it does not require laboratory monitoring or special dietary restrictions.
Contraindications
- hypersensitivity to apixaban or any of the excipients
- pregnancy and breastfeeding (not recommended)
- severe renal impairment → use Cockcroft-Gault formula!
- contraindicated with CrCl <15 mL/min (0.25 mL/s)
- clinically significant active bleeding
- lesions or conditions considered a significant risk factor for major bleeding
- current or recent gastrointestinal ulcer, presence of malignant tumors
- a recent brain or spinal cord injury
- a recent brain, spine, or eye surgery
- recent intracranial hemorrhage, known presence or suspected esophageal varices, arteriovenous malformations, vascular aneurysms, or severe
intraspinal or intracerebral vascular anomalies
- spontaneous or pharmacologic disorders of hemostasis
- liver disease
- all DOACs are contraindicated in patients with liver disease associated with coagulopathy and clinically apparent risk of bleeding
- use cautiously in patients with mild-to-moderate hepatopathy (Child-Pugh A/B)
- no dosage adjustment is required for patients with mild hepatic impairment
- concomitant therapy (especially with potent P-glycoprotein inhibitors)
- ketoconazole, itraconazole
- macrolides
- mechanical heart valve requiring VKAs
Mechanism of action
- direct, competitive, highly selective inhibitor of activated Factor Xa (fXa)
- inhibits free Xa bound in a prothrombinase complex with cofactor V and incorporated into the fibrin network of the coagulum ⇒ in contrast to indirect fXa inhibitors, sufficient blockade of the fibrin-bound factor is ensured and secondary activation of coagulation after thrombus lysis does not occur
- unlike indirect Factor Xa inhibitors (heparin, LMWH), the effect is not dependent on the presence of antithrombin III (AT III)
- up to 5% of the population with thrombotic complications have insufficient antithrombin levels
- the anticoagulant effect is directly proportional to the apixaban plasma levels and lasts only for this period
| Direct anticoagulants They inactivate the clotting factors present in the plasma |
Indirect anticoagulants They affect clotting factors by reducing their liver production |
|
| Direct thrombin/factor Xa inhibitors These drugs bind to thrombin/factor Xa and thereby block their function |
Indirect thrombin/factor Xa inhibitors These drugs activate antithrombin |
|
|
||
|
||
|
||
Pharmacokinetics and pharmacodynamics
Absorption and onset of action
- easily absorbed active drug
- apixaban has a bioavailability of 50-80% due to its low affinity for the P-glycoprotein transporter
- rapid onset of action (30-60 minutes), maximum concentration within 2-4 hours, apixaban half-life is ~ 13 hours ⇒ must be administered twice daily
- low inter-individual and intra-individual variability of effect due to reliable bioavailability, low variability of biotransformation and bioelimination
- little impact of renal or hepatic insufficiency
- minimal risk of food/drug interactions
Elimination
- 2/3 by the liver, 1/3 by the kidney
- apixaban is always partially degraded by CYP3A4/5
- can be administered to patients with mild-moderate renal impairment (up to 60% of Afib patients enrolled in the ARISTOTLE study had mild or moderate renal impairment)
- in patients with renal failure, exposure to apixaban is increased by approx. 1/4
apixaban is the substrate of two efflux pumps, P-gp and BCRP. The activity of both systems determines the bioavailability and bioelimination. Apixaban is also a substrate of CYP isoenzymes (mainly CYP3A4), influencing its transformation into inactive metabolites. Inhibition of P-gp increases bioavailability and slows the elimination into the bile; blockade of CYP3A4 slows inactivation and transformation. Many drugs are potent inhibitors of both CYP3A4 and P-gp. Clinically important inhibitors of both systems include azole-type antifungals, antiretrovirals, and the macrolides
Interactions
Pharmacodynamic interactions
- anticoagulants/antiplatelet agents/NSAIDs increase bleeding risk
- the addition of apixaban to ASA monotherapy or dual antiplatelet therapy significantly increases the risk of total and major bleeding (by 1-2% per year)
- temporal combination therapy is used in patients with recent coronary stenting and concomitant atrial fibrillation, etc.
Pharmacokinetic interactions
- interactions affect absorption, metabolism, or excretion
- the interaction potential of apixaban is very low; only prolonged combination with strong CYP3A4 and P-gp inhibitors should be avoided
- CYP3A4 and glycoprotein P inhibitors (up to 50% increase in effect)
- azole antifungals (fluconazole, ketoconazole)
- antiretrovirals (ritonavir)
- macrolide antibiotics (clarithromycin and erythromycin)
- weaker CYP3A4 and glycoprotein P inhibitors increase exposure only slightly (by ~ 30%)
- verapamil, diltiazem, amiodarone, grapefruit juice
- verapamil, diltiazem, amiodarone, grapefruit juice
- P-gp and CYP3A4 inducers (rifampicin, PB, PHE, CBZ) decrease the efficacy
- patients at risk:
- renal insufficiency (less important than with dabigatran)
- liver disease with coagulopathy – do not use DOACs
- concurrent use of NSAIDs, antithrombotics
- age > 85 years
- low body weight (~ 30% higher exposure)
- there is usually no significant clinical effect if only one of these factors is present. Dosage should be adjusted when they combine
Dosing
- taken orally twice a day (every 12 hours) with or without food
- the tablet may be crushed and mixed with water or apple juice as needed
| Content available only for logged-in subscribers (registration will be available soon) |
Monitoring
- clinical monitoring usual for all anticoagulated patients
- specific laboratory monitoring may be performed in selected cases
Complications, adverse events
Bleeding
- in the AVERROES trial, long-term treatment with apixaban 10 mg daily resulted in clinically significant bleeding and major bleeding in 4-5% of patients per year
- intracranial bleeding occurred in 0.5% of patients
- the most common bleeding events are epistaxis, GI bleeding, hematuria or bleeding at the surgical site, puncture, or catheter site
- the incidence of bleeding is lower compared to warfarin (relative reduction of 50%) [Agnelli, 2013]
Other adverse events
- anemia (1%) – often due to bleeding
- GI disorders (nausea)
- asymptomatic elevation of liver enzymes
- usually mild and transient
- usually mild and transient
- mild transient thrombocytopenia
- other adverse effects are mild and rare
Overdose
- if the agent was administered within the last 2 hours, consider activated oral charcoal (recommendation with no proven clinical benefit)
- there is no hard evidence regarding the antifibrinolytics (tranexamic acid, aminocaproic acid) or systemic hemostatic agents (desmopressin, aprotinin)
- the antidote is still somewhat a theoretical possibility (price, availability) → andexanet alfa
- andexanet alfa (sold under the trade name Andexxa) is an antidote for rivaroxaban and apixaban when the reversal of anticoagulation is needed. It has not been found to be useful for other Factor Xa inhibitors
- both antidote and substitution therapy should be reserved for significant bleeding or urgent surgery (always consider ↑ risk of VTE)
FAQs
- apixaban is an oral anticoagulant used to prevent and treat blood clots
- it’s commonly prescribed for conditions like atrial fibrillation (to reduce the risk of stroke), deep vein thrombosis (DVT), and pulmonary embolism (PE)
- apixaban is a direct inhibitor of Factor Xa
- this inhibition reduces the formation of clots, thereby lowering the risk of stroke or other clot-related events
- routine monitoring of coagulation tests is not required; however, specific tests are available if required
- kidney function should be tested before starting treatment and periodically thereafter, especially in patients with renal impairment
- apixaban does not have significant dietary restrictions like warfarin, but patients should maintain a consistent lifestyle
- common side effects include bleeding (like nosebleeds or heavier menstrual bleeding), bruising, nausea, and allergic reactions. Severe bleeding is a significant risk, especially in patients with a history of bleeding disorders.
- apixaban can be used in patients with mild to moderate renal impairment, but dosage adjustments may be necessary
- it should be used with caution in severe renal impairment and is not recommended for patients with end-stage renal disease on dialysis
- if a dose is missed and < 6 hours have passed, take it as soon as remembered
- if > 6 hours have passed, skip the missed dose and continue as usual
- do not double the dose
Pregnancy:
-
- apixaban is a pregnancy category B drug – animal reproduction studies have not shown a fetal risk, but there are no adequate and well-controlled studies in pregnant women
- it should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- there is a potential risk of bleeding in both the mother and fetus
Lactation:
-
- It is not known if apixaban is excreted in human breast milk
- a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother
- if a nursing mother requires anticoagulation, alternatives to apixaban that have more established safety profiles during breastfeeding may be considered