ISCHEMIC STROKE / CLASSIFICATION AND PATHOGENESIS
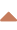
Pulmonary A-V malformation
Updated on 03/03/2024, published on 31/10/2021
- pulmonary arteriovenous malformation (PAVM) comprises abnormal connections between pulmonary arteries and veins
- synonyms: pulmonary AVM, pulmonary arteriovenous fistula, pulmonary arteriovenous aneurysm
- PAVMs are rare in the general population
- it is estimated that 70-80% of PVAMs are related to HHT (hereditary hemorrhagic telangiectasia – Osler-Weber-Rendu), mainly HHT1 (10 times more than HHT2) → more about HHT see here
- ≥ 15% of patients do not fulfill the criteria for a diagnosis of HHT and have no other systemic disease
- neurological complications occur in ~ 50% of patients
- paradoxical embolism can cause TIA/stroke, ICH, or a cerebral abscess
- concurrent cerebral AVM (CAVM) can cause ICH or epilepsy
Pathophysiology
- direct arterio-venous shunts without embedded capillaries
- PAVM, together with PFO, patent ductus arteriosus, and other congenital heart defects, is categorized as a condition at risk of paradoxical embolism
- contrary to PFO, where the shunt is frequently seen only post-Valsalva maneuver, the shunt in PAVM is continuous
- concurrent occurrence of PAVM and PFO is possible (consider the possibility of PAVM before PFO closure)
- disproportionately massive and early shunt on the TCCD bubble test in the case of a small/moderate PFO on TEE should raise suspicion of another shunt
- additional pathogenetic mechanisms besides paradoxical embolism include:
- polycythemia with hyperviscosity syndrome
- hypoxia
- air embolism originating from a defect in the PAVM wall
- polycythemia with hyperviscosity syndrome
- PAVMs may be located in any lung segment, with a predilection for the lower lobes
- caused by any right-to-left shunt (RLS)
- patent foramen ovale (PFO)
- pulmonary arteriovenous malformation
- patent ductus arteriosus
- iatrogenic communications
- atrial/ventricular septal defects
- patent foramen ovale (PFO)
- embolus type:
- thrombus
- fat
- air
- amniotic fluid
- tumor tissue
Classification
- according to the feeding artery
- simple (2/3) – single feeding artery with a single draining pulmonary vein
- complex (1/3) – multiple feeding arteries or draining veins
- diffuse type (~5%) – dozens to hundreds of malformations
- by other symptoms
- isolated PAVM
- PAVM in HHT (together with telangiectasias)
Clinical presentation
- generally slow progression, with potential acceleration during pregnancy and adolescence
- the clinical course differs between isolated PAVM and PAVM associated with HHT
- symptoms most commonly occur between the 3rd and 6th decades of life
- an asymptomatic course is observed in up to 50% of patients (mostly in PAVM < 2cm)
- mortality and morbidity are mostly associated with stroke and brain abscess, less commonly with hypoxemic respiratory failure, hemoptysis, or hemothorax
Neurological complications
| Content available only for logged-in subscribers (registration will be available soon) |
|
1. epistaxis, recurrent |
2. telangiectasia
|
3. visceral lesions
|
4.positive family history |
|
definitive diagnosis: ≥3
probable diagnosis: 2 criteria diagnosis unlikely: < 2 criteria |
|||
Diagnostic evaluation
TCCD
- good screening method to detect right-sided shunt (usually a massive shunt is present)
Contrast transthoracic echocardiography (cTTE)
- recommended as the screening test of choice for PAVMs in HHT – sensitivity 94-100%, specificity 80%
- after administration of agitated saline solution, microbubbles typically appear in the atrium within 3-8 cardiac cycles
- in septal shunts, contrast is visible almost immediately
- grading (significant association between TTCE grade and presence of PAVMs on CT was found) [Zukotynski, 2007]
- grade 1 – minimal opacification of the left ventricle
- grade 2 – moderate opacification
- grade 3 – extensive opacification without endocardial outlining
- grade 4 – extensive opacification with endocardial definition
- grade 1 – minimal opacification of the left ventricle
CT+CTA / MRI+MRA
- non-contrast +/- contrast-enhanced
- CE-MRA is suitable for screening; it enables accurate detection and staging of pulmonary AVMs, and differentiates lesions requiring embolization
- limitations exist in detecting PAVMs <5 mm
- CT
- high sensitivity (comparable to DSA)
- use of thin-slice (2-3 mm) non-contrast CT with 3-D reconstruction is recommended
- the benefit of performing a contrast-enhanced CT pulmonary angiogram must be weighed against the risk of introducing air, leading to paradoxical embolism
Digital subtraction angiography
Chest X-ray
- an initial imaging modality for patients presenting with hypoxemia or hemoptysis
- chest X-ray has low sensitivity (70%) and is not ideal for screening
- non-specific soft tissue mass with uniform density can be detected in larger PAVMs
Management
- therapy targets:
- prevent neurological complications
- prevent pulmonary hemorrhage
- improve hypoxemia, which can lead to fatigue, weakness, and other health problems
- indications for invasive management:
- progressive PAVM growth (which indicates an increasing risk of complications)
- paradoxical embolization (when blood clots or other debris travel through the PAVMs to the brain, causing strokes)
- symptomatic hypoxemia
- feeding vessels ≥3 mm
- no specific medical treatment
Endovascular treatment
| Content available only for logged-in subscribers (registration will be available soon) |
Surgical treatment
- lobectomy or pneumonectomy are rarely performed
- thoracoscopic procedures are preferred, especially in short-feeding arteries with an increased risk of coil migration
Thrombolysis in patients with PAVM
- no clear evidence in this population ⇒ no recommendations are available
- high risk of complications with PAVM in HHT ⇒ prefer direct mechanical thrombectomy
- in isolated PAVM, the risk is significantly lower ⇒ intravenous thrombolysis may be considered [Lin, 2019]
Prevention
- lifetime antibiotic prophylaxis is recommended for surgical and dental procedures
- extreme caution is necessary during intravenous administrations to prevent air embolism
- in patients with known untreated PAVM, a follow-up CT scan every 2-3 years and annual oximetry are advised
- exclude cerebral AVM (CAVM) in patients with PAVM; evaluation of hepatic AVM is not routinely performed
- annual follow-ups are performed after endovascular treatment
- screening in offspring of parents with HHT includes:
- pulse oximetry every 1-2 years
- O2 saturation < 97% warrants a TTE
- in the presence of TTE abnormalities, a chest CT is indicated
Antithrombotic medication in stroke prevention
- in HHT patients, antithrombotic therapy is not absolutely contraindicated; however, careful monitoring is required [Gaetani, 2020] [Garg, 2014]
- higher risk of bleeding is associated with anticoagulant therapy
- after a stroke or myocardial infarction, the benefit of antiplatelet therapy is likely to outweigh the risk of bleeding