ISCHEMIC STROKE / ETIOLOGY
Grange syndrome
Updated on 14/01/2024, published on 31/10/2022
Etiology
- Grange syndrome results from mutations in the YY1AP1 gene
- homozygous or compound heterozygous mutations in the YY1AP1 gene, which is located on chromosome 1q22, are associated with this condition
- parents of an individual with an autosomal recessive condition carry one copy of the mutated gene but typically do not exhibit signs and symptoms of the syndrome
- homozygous or compound heterozygous mutations in the YY1AP1 gene, which is located on chromosome 1q22, are associated with this condition
- the YY1AP1 gene encodes a protein that plays an important role in regulating smooth muscle cell (SMC)
- mutations lead to the cell cycle arrest with reduced proliferation and differentiation of SMC
- the exact pathway linking these cellular changes to vascular narrowing remains unclear
- it is also unknown how YY1AP1 gene mutations are related to other features, such as bone abnormalities
Clinical presentation
Diagnostic evaluation
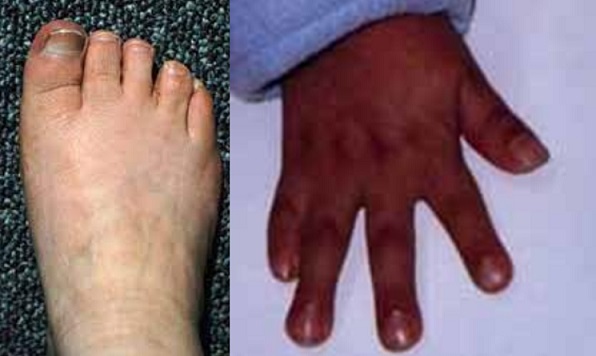
- early-onset vasculopathy with brachy- and syndactyly and bone fragility are characteristic features of this syndrome
- oligosymptomatic forms, such as isolated intracranial stenoses, pose a diagnostic challenge
Vascular imaging (CTA, MRA, DSA)
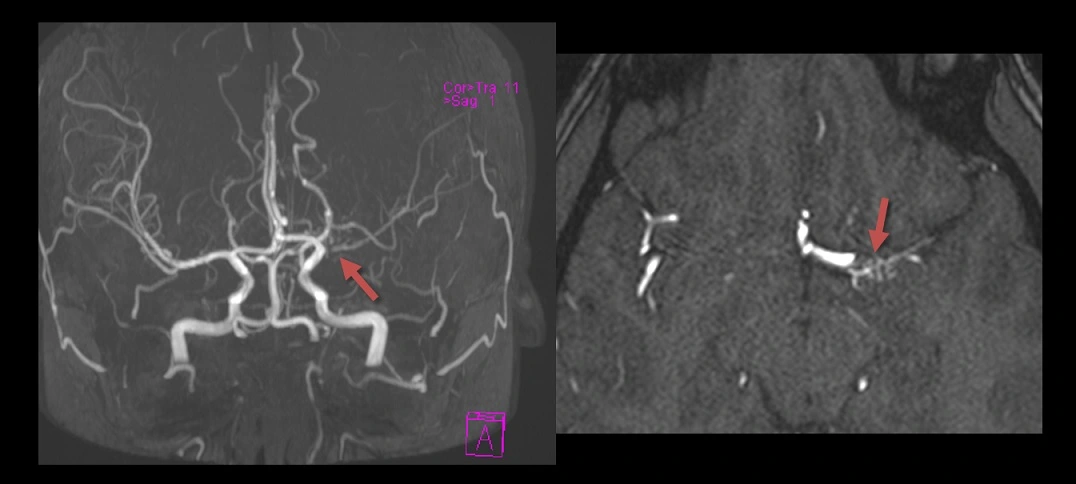
- stenoses of the internal carotid arteries and MCAs + collateral vessel formation ⇒ moyamoya-like pattern
- signs of inflammation are absent on black blood sequences
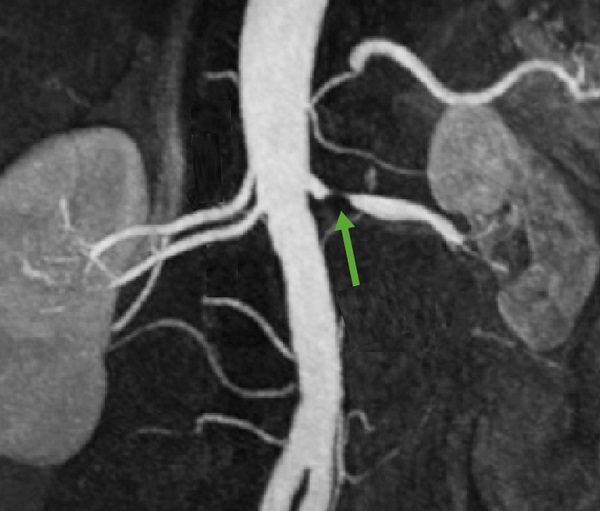
- stenosis of renal, celiac, or coronary arteries (can be assessed using US, CTA, DSA, MRA)
Cardiac monitoring
- regularly check ECG as prolonged QT interval has been reported in Grange syndrome
- perform TTE to assess for valvular abnormalities and cardiomyopathy
Genetic testing
- collect DNA samples to identify pathogenic YY1AP1 variant
Management
- currently, no causal therapy is available
- administer antiplatelet therapy if stroke or TIA symptoms occur
- manage vascular risk factors
- consider angioplasty of carotid, renal or celiac stenoses
- consider intracranial revascularization procedures in selected cases (as with moyamoya)
- genetic counseling