ISCHEMIC STROKE / CLASSIFICATION AND ETIOLOGY
CADASIL
(Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy)
Updated on 26/07/2024, published on 11/05/2023
- Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a hereditary disease characterized almost exclusively by neurological manifestations (premature small vessel disease, migraines, etc.)
- it typically affects individuals over the age of 30 (onset usually occurring between 40 and 50 years of age); life expectancy is reduced in people with CADASIL.
- the phenotype is highly variable, and although the full clinical and neuroimaging presentation may be indicative of the disease, genetic testing or skin biopsy is necessary to confirm the diagnosis
- once diagnosed, genetic counseling should be offered to affected individuals and their families
Definition and pathophysiology
- CADASIL is the most common small vessel disease caused by a single gene mutation
- the responsible gene was discovered by Joutel et al. in 1996
- mutations in the NOTCH 3 gene are located on chromosome 19 – exons 2-24; > 200 mutations have been identified so far
- the NOTCH3 protein is a transmembrane receptor with extracellular epidermal growth factor (EGF) repeat domains
- CADASIL is inherited in an autosomal-dominant (AD) manner, meaning that only one copy of the mutated gene is required for the disease to be passed down from a parent to their child
- mutations in the NOTCH 3 gene are located on chromosome 19 – exons 2-24; > 200 mutations have been identified so far
- the mutation causes the progressive degeneration of smooth muscle cells of small cerebral vessels due to accumulation of NOTCH 3 in the basal membrane (visible as osmiophilic granules in electron microscopy) ⇒ impaired autoregulation, hypoperfusion, ischemia
- although angiopathy is generalized and can be detected through skin biopsy, CADASIL is characterized by almost exclusively neurologic manifestations
Clinical presentation
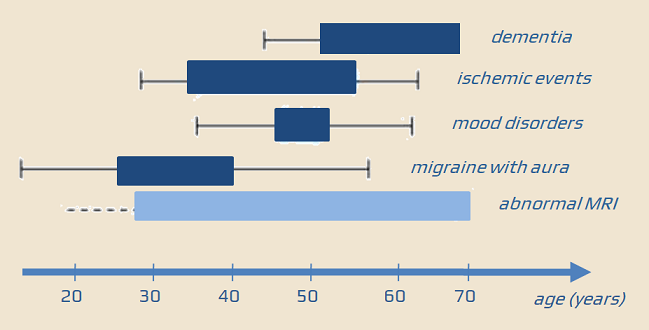
- migraine (usually with aura) is the most common initial symptom, typically presenting between the ages of 20 and 30 (present in ~ 55% of cases)
- TIA or strokes occur between the ages of 30 and 45, accompanied by “clinically asymptomatic” lacunar infarcts and white matter lesions (WMLs)
- progressive subcortical lacunar infarcts and WML are the basis for the development of neuropsychiatric symptoms
- mood disorders (depression, apathy)
- impaired psychomotor speed and executive function
- progressive cognitive impairment leading to dementia and disability
- additional symptoms may occur depending on the number and location of infarcts
- paresis
- ataxia
- pseudobulbar syndrome
- urinary incontinence
- visual and speech disturbances
- in some cases, acute reversible encephalopathy (sometimes accompanied by epileptic seizures) may occur, with spontaneous resolution and recurrence [Schon, 2003]
- clinical presentation of CADASIL may be extremely variable, with some patients exhibiting minimal disturbances and a milder course even at an advanced age
Diagnostic evaluation
- CADASIL should be considered in individuals with a strong family history of early-onset stroke, dementia, and typical MRI findings
- a CADASIL scale (see further) has been proposed to guide the selection of appropriate individuals for genetic testing or skin biopsy
- definitive diagnosis typically involves serum genetic testing for NOTCH3 mutations
- for individuals with negative genetic testing, the presence of unidentified genetic mutations may warrant a skin biopsy with histopathologic examination to assess for granular osmiophilic material (GOM) accumulation
Personal history
- typically early-onset TIA/stroke, often occurring in the absence of traditional vascular risk factors
- gradual progression of cognitive impairment, leading to dementia
- migraine with aura
- positive family history
Neuroimaging
- CT shows nonspecific hypodensities in the white matter (leukoaraiosis)
- MR is crucial in the diagnosis of inherited CSVDs
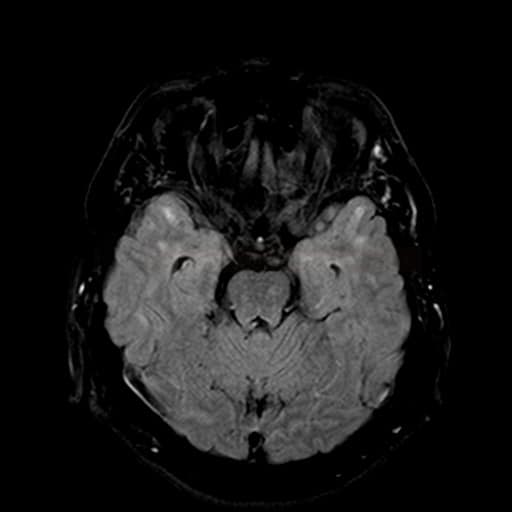
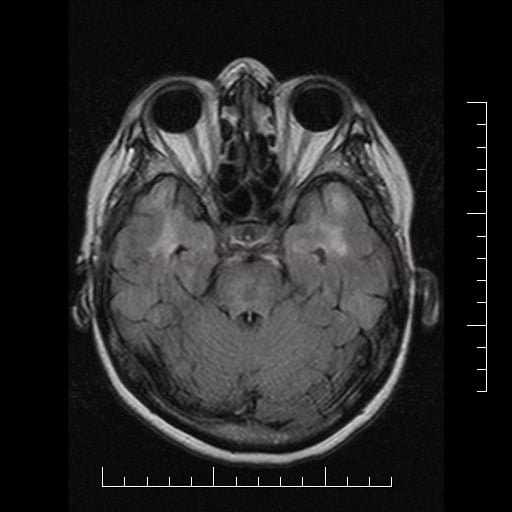
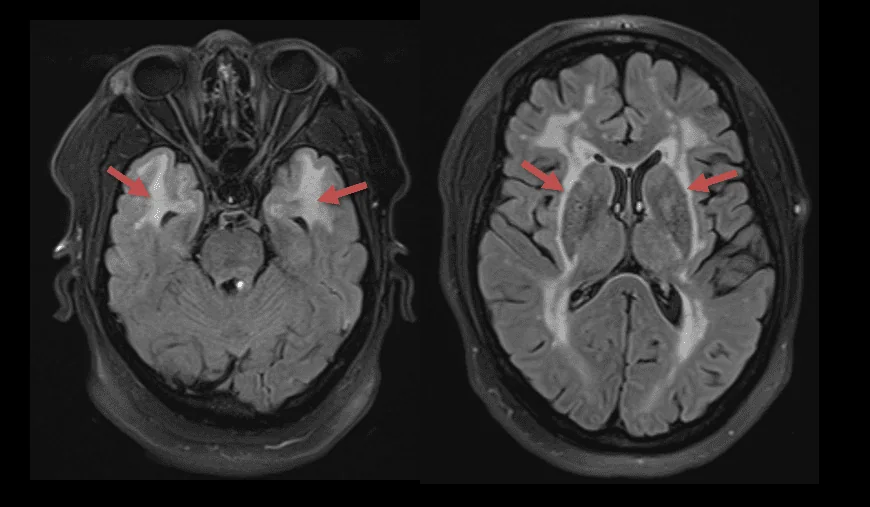
- white matter hyperintensities (lesions) in T2/FLAIR [Stojanov, 2015]
- subcortical lacunar infarcts (basal ganglia, periventricular white matter, and pons)
- cerebral microbleeds (CMBs) → see here
- best seen on GRE or SWI sequences as multiple punctate foci of susceptibility/hypointensity
- dilated perivascular spaces (T2-weighted MRI)
- brain atrophy
Neurosonology
- intracranial flow is decreased along with decreased cerebral vasomotor reactivity (CVR) [Pfefferkorn,2001] [Engelter, 2002]
Laboratory tests
- genetic testing – the gold standard for definitive diagnosis of CADASIL
- negative results may be attributed to the presence of a novel NOTCH3 mutation
- skin biopsy (systemic arteriolopathy) – it is necessary to obtain subcutaneous tissue with blood vessels; the sample should be fixed in glutaraldehyde
- electron microscopy – granular osmiophilic material (GOM) in the vessel wall
- immunohistochemistry – detection of intravascular deposits in the smooth muscle of the vessel wall using molecular antibodies against NOTCH3 [Joutel, 2001]
- skin biopsy is highly specific but exhibits variable sensitivity
The CADASIL scale is a simple and sufficiently accurate screening tool to select patients with a high probability to be affected by the disease and, therefore to be subjected to the genetic testing
|
Diagnosis is possible
Diagnosis is probable
Diagnosis is certain
|
| CADASIL Scale [Pescini, 2012] Max 25 points, a total score of ≥15 predicts CADASIL diagnosis |
|
| Migraine | 1 |
| Migraine with aura | 3 |
| TIA/stroke | 1 |
| TIA/stroke onset <50 y | 2 |
| Psychiatric disturbances | 1 |
| Cognitive decline/dementia | 3 |
| Leukoencephalopathy | 3 |
| Leukoencephalopathy extending to the temporal pole | 1 |
| Leukoencephalopathy extending to the external capsule | 5 |
| Subcortical infarcts | 2 |
| Family history* in at least 1 generation | 1 |
| Family history* in at least 2 generations | 2 |
| * at least 1 of the typical disturbances (headache, transient ischemic attack/stroke, cognitive decline, psychiatric disturbances) | |
- the gold standard for confirming the diagnosis (may be positive in biopsy-negative patients)
- careful selection of patients (e.g., using the CADASIL score above) is required
- the analysis of the NOTCH3 gene is costly and time-consuming
- the negativity of the test does not exclude other inherited diseases; therefore, a negative genetic test does not provide complete reassurance for patients
- genetic testing may sometimes be positive in biopsy-negative patients
- genetic counseling is needed in case of a positive result
- genetic counselors can provide detailed information about the disease, its inheritance pattern, and the risk of passing it on to offspring
- they can also assist families in making informed decisions about their healthcare and reproductive choices
- a significant number of patients presenting with a phenotype suggestive of CADASIL have no pathogenic mutations on exons 2–23 of the NOTCH3 gene
- this observation highlights the possibility that the genetic spectrum of the CADASIL phenotype may be broader than previously recognized
- novel NOTCH3 mutations
- a new mutation on exon 24 has been reported (outside the traditional exon 2-23 region) in a patient with CADASIL-like features
- a novel activating mutation on exon 25 has been described in a patient with cerebral SVD but without GOM deposits and NOTCH3 receptor accumulation (genetic testing may be a more reliable method for diagnosing)
- other genes causing CADASIL-like hereditary cerebral SVD (NOTCH3-negative)
- novel NOTCH3 mutations
Differential diagnosis
- Binswanger’s disease
- typically affects elderly individuals with multiple vascular risk factors and systemic atherosclerosis
- absence of typical temporal lobe lesions
- CARASIL (Cerebral Autosomal Recessive Arteriopathy with Subcortical Infarcts and Leukoencephalopathy)
- mutation of HTRA1 serine peptidase gene on chromosome 10q (10q25.3-q26.2)
- early onset, no migraine
- clinically similar to CADASIL + early alopecia and spondylosis deformans
- no GOM granules in the skin biopsy
- arteriolopathic changes on MRI + arc sign (hyperintensity connecting peduncles across the pons in T2-weighted images)
- AD form with one pathologic allele (HTRA1-related disease) has a milder presentation and less pronounced MRI lesions compared to the fully penetrant form
- Susac syndrome
- no temporal lobe involvement
- characterized by retinopathy and hearing impairment
- MELAS
- caused by mitochondrial dysfunction and is associated with recurrent stroke-like episodes, seizures, and lactic acidosis
- primary angiitis of the CNS (PACNS)
- positive biopsy, wall enhancement on MRI black blood sequences
- COL4A1/2 related cerebral small-vessel diseases (CSVD) – genetic disorders that affect the collagen IV proteins
- HANAC syndrome (hereditary angiopathy with nephropathy, aneurysms, and muscle cramps) (Alamowitch, 2009)
- PADMAL (pontine autosomal dominant microangiopathy with leukoencephalopathy)
- CARASAL (cathepsin A-related arteriopathy with strokes and leukoencephalopathy) – AD inheritance, very rare
- Fabry disease
- an X-linked disorder caused by a deficiency in α-galactosidase A, an enzyme responsible for degrading glycosphingolipids
- albuminuria, angiokeratomas, renal disease, cardiomyopathy, and autonomic and painful peripheral neuropathies
- RVCL-S (retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations)
- TREX1 gene mutation (Wilms, 2022)
- characterized by retinal and CNS vascular changes, as well as systemic manifestations
- TREX1 gene mutation (Wilms, 2022)
- Behcet’s disease
- acute disseminated encephalomyelitis
- HIV encephalopathy and AIDS dementia complex
- Lyme disease
- multiple sclerosis
- different MRI appearance and involvement of the optic nerves and spinal cord ( not be expected in CADASIL)
- neurosarcoidosis
- neurosyphilis
Management
Medication
- at present, there is no causal or specific disease-modifying treatment available; therapy is similar to that for traditional small vessel disease
CADASIL and perioperative managament
- patients with CADASIL may be at an increased risk of perioperative stroke or delirium (PET studies have shown a significant decrease in cerebral blood flow in white matter, corresponding to WMHs)
- to minimize these risks, it is suggested to:
- maintain intraoperative mean arterial pressure (MAP) >60 mmHg (8 kPa)
- keep end-tidal carbon dioxide at ~ 40 mmHg (5.3 kPa)
- avoid or restrict head-down positioning during surgery
CADASIL and pregnancy
- CADASIL is probably not associated with higher rates of complications during pregnancy, peripartum, or postpartum period
- according to the European Guidelines 2020, antithrombotics are not routinely recommended during pregnancy
- no evidence suggesting that vaginal birth is unsafe for women with CADASIL