MEDICATION / ANTICOAGULANT THERAPY
Warfarin
Updated on 24/01/2024, published on 07/11/2022
- vitamin K antagonists (VKAs) are indirect oral anticoagulants that have been available since the 1950s and were commonly used for VTE prophylaxis and prevention of cardioembolic strokes
- nowadays, wherever possible, VKAs are being replaced by DOACs (Direct Oral Anticoagulants)
Mechanism of action
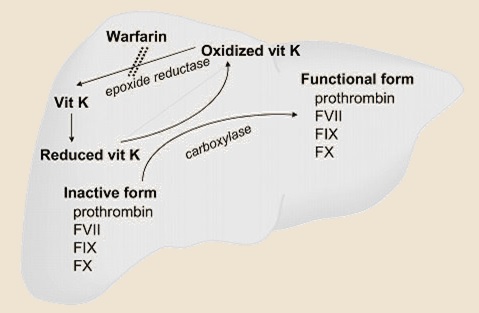
- warfarin is an indirect oral anticoagulant drug belonging to vitamin K antagonist (VKA), also known as coumarins
- warfarin inhibits vitamin K epoxide reductase complex 1(VKORC1) ⇒ depletion of reduced vitamin K (KH2) ⇒ liver cells produce hypofunctional (non-carboxylated) clotting factors II, VII, IX, and X (vitamin K-dependent clotting factors), as well as the the regulatory proteins C and S
- the coagulation factors are normally activated via carboxylation
- vitamin K1 in food can reverse the effects of coumarins because it is reduced to vitamin KH2 by a warfarin-insensitive vitamin K reductase
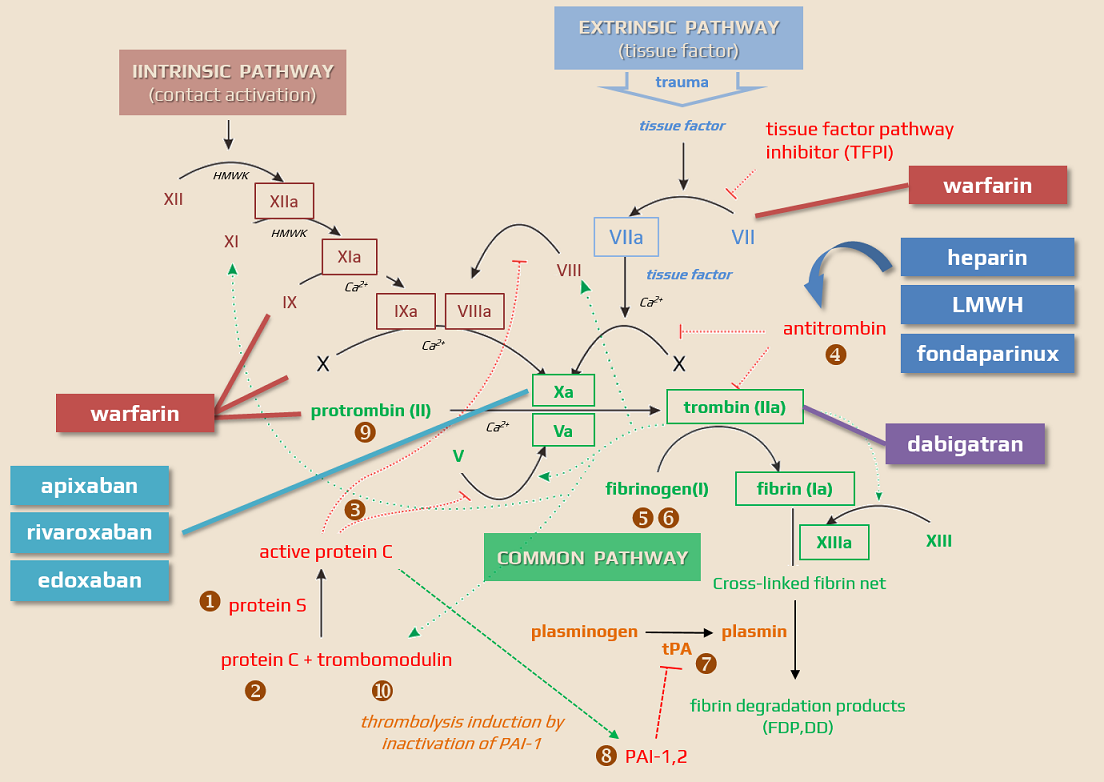
| Direct anticoagulants They inactivate the clotting factors present in the plasma |
Indirect anticoagulants They affect clotting factors by reducing their liver production |
|
| Direct thrombin/factor Xa inhibitors These drugs bind to thrombin/factor Xa and thereby block their function |
Indirect thrombin/factor Xa inhibitors These drugs activate antithrombin |
|
|
||
|
||
|
||
Indications
Main indications (in most of them, warfarin has been replaced by DOACs):
- prevention of cardioembolic stroke
- in uncomplicated NVAF prefer DOACs
- use warfarin if direct oral anticoagulants (DOACs) are contraindicated or not recommended
- therapy and prevention of cerebral venous thrombosis
- dabigatran can be used instead of warfarin
- warfarin should be used in patients with a major hypercoagulable state
- arterial dissection
- usually replaced by DOACs or dual antiplatelet therapy
- VTE prophylaxis and treatment (venous thrombosis/pulmonary embolism)
- warfarin is preferred to DOACs in patients with hypercoagulable state or if DOACs are contraindicated
- warfarin is preferred to DOACs in patients with hypercoagulable state or if DOACs are contraindicated
VKAs, such as warfarin, are preferred in certain clinical situations due to specific considerations and limitations of DOACs
- mechanical heart valve – DOACs are not recommended due to a higher risk of thromboembolic events and bleeding in this group
- AFib + moderate to severe mitral valve stenosis
- Afib + intracardiac thrombus (increased risk of stroke or systemic embolism with DOACs)
- Afib + hypertrophic cardiomyopathy (HCM) (limited data with DOACs)
- Afib + severe aortic valve stenosis (limited data with DOACs)
- severe renal impairment – e.g., creatinine clearance less than 15-30 ml/min, warfarin is often preferred because most DOACs are contraindicated or require cautious use
- antiphospholipid syndrome – particularly in patients with triple-positive antiphospholipid syndrome (lupus anticoagulant, anticardiolipin antibodies, and anti-beta-2-glycoprotein I antibodies), warfarin is favored as DOACs may be less effective and carry a higher risk of recurrent thrombotic events.
- pregnancy and lactation
- warfarin and DOACs are contraindicated during pregnancy due to teratogenic effects
- while DOACs are also generally avoided in lactation, warfarin may be a safe alternative postpartum
- extremes of body weight – warfarin might be preferred due to concerns about the efficacy and safety of DOACs in these populations
- drug interactions – if a patient is on medications that have significant interactions with DOACs, particularly those that affect the CYP3A4 and P-glycoprotein pathways, warfarin might be a safer choice
- cost considerations – in some regions, warfarin may be more cost-effective than DOACs
- according to meta-analyses, warfarin reduces the annual risk of stroke in Afib patients by 64% compared to placebo and by 39-46% compared to aspirin
[Hart, 2007] [Walraven, 2002]
- according to the above-stated meta-analysis, the absolute increase in major bleeding was ~ 0.9-1.5% per year
- ASA is not an alternative in Afib patients
- warfarin is also more effective than dual antiplatelet therapy (ASA+CLP) (ACTIVE W trial)
- the SPAF III (Stroke Prevention in Atrial Fibrillation) trial did not show superior efficacy of low-dose warfarin (INR 1.2-1.5) in combination with ASA compared to full-dose warfarin (with INR 2-3)
- generally, the combination of warfarin + ASA is not recommended in Afib
- according to meta-analyses, it leads to a reduction of ischemic stroke only in patients with mechanical valves
- this combination can be considered in patients with additional risk factors (e.g., CAD, ACS, etc.)
Contraindications
- conditions with a high risk of bleeding
- pregnancy and puerperium (switching to LMWH is required) because warfarin crosses the placenta, which may result in:
- fetal embryopathy (nasal hypoplasia and epiphyseal damage), with the risk being greatest between 8-12 weeks gestation
- neurotoxicity
- an increased risk of bleeding
- warfarin can be used during breastfeeding as it does not pass into breast milk
Pharmacokinetics and pharmacodynamics
- warfarin exhibits rapid and complete absorption from the gut (food may prolong the absorption time but does not reduce the absorption rate)
- the onset of action typically occurs within 24-72 hours; a peak therapeutic effect is seen 5-7 days after initiation
- duration of action: 2-5 days
- it has relatively long elimination half-life
- 20-60 hours; mean 40 hours
- half-life is highly variable among individuals
- circulates in the blood bound to plasma proteins (mainly albumin – 90%) and accumulates in the liver, where its isomers are metabolized
- only free, unbound VKA is biologically active
- warfarin is administered as a racemic mixture of R and S enantiomers
- S-warfarin has a shorter half-life (approx. 33 hours) but is 4-5x more effective (70% effect) than the R-isomer (whioch has half-life ~ 45 hours)
- S-warfarin concentration has a more significant impact on the efficacy of anticoagulation
- metabolised primary in the liver through the CYP2C9 enzyme; other minor enzymatic pathways include CYP2C8, 2C18, 2C19, 1A2, and 3A4
- the metabolites are excreted through the kidneys (~ 92%); a smaller portion of the metabolites may also be eliminated through the feces
- warfarin itself is not excreted unchanged to a significant extent
- there are significant interindividual differences in the pharmacokinetics and pharmacodynamics of warfarin ⇒ the dose should be strictly individualized
- interindividual variability results in a wide range of effective doses
- slow metabolizers (warfarin-sensitive) require a dose of ~ 1 mg per day
- fast metabolizers (warfarin-resistant) need a dose above 10 mg per day
| Content available only for logged-in subscribers (registration will be available soon) |
Interactions
- warfarin has interactions with vitamin K-containing foods (sucha s leafy vegetables), herbal suplements and drugs
| Content available only for logged-in subscribers (registration will be available soon) |
Dietary precautions during warfarinization
- warfarin has interactions with vitamin K-containing foods (such as leafy vegetables)
- the aim is not to exclude vegetables and fruit from the diet but to maintain a stable, healthy diet without large fluctuations
- vitamin K is also present in meat; its content depends on how the animal is fed. In summer, animals contain more vitamin K in their meat than in winter
- substances used in alternative medicine can affect warfarin levels
- St. John’s wort, ginseng, and garlic can reduce warfarin concentration
- ginkgo may increase warfarin concentration
- patients should be advised that anticoagulation levels should be checked more frequently when when making a major long-term change in a diet high in leafy vegetables
Low content (< 10 μg/100 mg)
|
Medium content (10-40 μg/100 g)
|
|
high content ( > 40 μg/100 g)
|
Dosing and monitoring
- usually, the treatment starts with a dose of 1.5-5 mg/day (most commonly with LMWH bridging), allowing a gradual onset of the effect
- do not start with high doses as they can lead to a rapid decline in protein C and S ⇒ ↑ risk of hypercoagulable state and warfarin-induced skin necrosis
- a higher dose increases the risk of INR overshoot (especially in CYP2C9 and VKORC1 variants) ⇒ ↑ risk of hemorrhage
- the dose can be quite precisely calculated and adjusted based on pharmacogenetics (which is usually not available)
- LMWH should be discontinued once the INR is >2
- timing
- taken as a once-daily oral medication
- it is recommended to administer warfarin in the afternoon or evening to allow for same-day adjustment of the patient’s warfarin dose based on current lab values
- frequency of INR checks
- initially, check INR every day (every other day during the next week)
- then once a week until dose stabilization (with 3 consecutive INR 2-3)
- long-term monitoring with a stabilized INR typically involves checks once per month, with self-monitoring being beneficial
- the usual maintenance dose is 1.5-10 mg/d
- it is preferred to maintain a stable daily dose or make minimal day-to-day dose variations
- significant alternate dosing leads to INR fluctuations, especially in borderline situations like infection, dietary errors, etc.
- educate patients about the need for earlier monitoring in high-risk situations:
- concurrent diseases
- changes in medication (check interactions!)
- increase in minor bleeding
- difficulty in maintaining the therapeutic INR range is most often caused by:
- warfarin interactions with certain foods and drugs (e.g., broccoli, spinach, kale)
- genetic factors
- poor adherence to treatment
- starvation (resultin in low albumin) or reduction diets
- excessive alcohol consumption
| Content available only for logged-in subscribers (registration will be available soon) |
Long-term monitoring
| Content available only for logged-in subscribers (registration will be available soon) |
Warfarin and renal insufficiency
- consider the renal risks associated with VKA treatment and specific complications that may arise in patients with advanced chronic kidney disease (CKD)
- patients with CKD are at higher risk of drug-drug interactions and are also at higher risk of warfarin-related nephropathy (WRN)
- WRN is characterized by the sudden progression of renal insufficiency, marked by glomerular vascularization and the presence of erythrocyte cylinders in the renal tubules
- more common in patients with renal dysfunction but it has also been reported in individuals with normal renal function.
- other specific complications of warfarin use in CKD patients include the effect on bone metabolism ⇒ calcification in large arteries and valves, which may prevent later renal transplantation
- CKD patients treated with warfarin are also at risk of coumarin-induced skin necrosis (CISN) and calcific uremic arteriolopathy (CUA), also known as calciphylaxis; which has a high mortality rate and is often underdiagnosed and inadequately treated
- in dialysis patients, warfarin administration an exacerbate adynamic bone disease
- on the other hand, warfarin is still the only option for oral anticoagulation in patients with a glomerular filtration rate < 15 mL/min/1.73 m2 (0.25 mL/s/1.73 m2), which corresponds to CKD stage 5
- LMWHs are an alternative – their advantage is a well-predictable effect and relatively high safety; the disadvantage is the need for parenteral administration
Adverse events
- bleeding is the most common complication (5-10%) associated with warfarin therapy; the annaul risk is individual → risk and prevention of bleeding in anticoagulant therapy
- patients with renal insufficiency and hepatopathy are at higher risk
- according to studies, the majority of bleeding occurs with INR in the therapeutic range (EHRA 2018)
- it is necessary to distinguish between major and minor bleeding
- major bleeding typically includes intracranial bleeding, retroperitoneal bleeding, and bleeding that necessitates the transfusion of ≥ 2 units of blood
- the most common cause of fatal bleeding is ICH
- mild/minor bleeding encompasses epistaxis, skin hematomas, suffusions, and micro/macroscopic hematuria
|