ISCHEMIC STROKE / RECANALIZATION THERAPY
Fibrinolytic drugs
Updated on 24/07/2024, published on 26/10/2021
- blood clotting is a fundamental homeostatic mechanism
- the participation of different systems is necessary:
- vascular wall (vasoconstriction)
- platelets (adhesion → activation → aggregation) with the formation of a primary plug
- plasma coagulation factors → thrombin formation → fibrin formation → definitive plug
- plasma inhibitors
- fibrinolytic system
Timing of successive steps of hemostasis:
- primary hemostasis:
- vasoconstriction (< 1 second)
- platelet adhesion and activation (several seconds)
- platelet aggregation and primary plug formation (seconds to minutes)
- coagulation:
- activation of coagulation factors (seconds to minutes)
- formation of a solid fibrin coagulum (minutes)
- fibrinolysis:
- activation of fibrinolysis (minutes)
- dissolution of fibrin coagulum (hours to tens of hours)
Fibrinolysis
- the fibrinolytic system has two functions in the process of hemostasis:
- dissolves the fibrin plug (within hours)
- limits excessive clotting
- the fibrinolytic system comprises a group of activators and inhibitors linked by a series of positive and negative feedback loops, with four main components:
- plasminogen
- plasmin
- plasminogen activators
- plasminogen inhibitors
- the main component is the inactive proenzyme plasminogen, which is a precursor of plasmin protease
- plasmin hydrolytically cleaves fibrin to form degradation products
- several plasminogen activators activate plasminogen to plasmin:
- tissue-type plasminogen activator (tPA) is mainly involved in the lysis of thrombi in the circulation
- urokinase-type (uPA) is involved in extravascular proteolysis
- plasminogen activation can also be mediated by streptokinase (a product of bacteria) or other fibrinolytics, kallikrein, and factor XIIa
- inhibition of fibrinolysis is crucial for maintaining hemostatic balance and preventing excessive clot breakdown, which could lead to bleeding
- plasminogen activator inhibitors (PAI-1 and PAI-2) – primarily target and inhibit tPA and uPA, thereby reducing the conversion of plasminogen to plasmin
- α2-antiplasmin – a potent inhibitor of plasmin, which binds to plasmin and inhibits its fibrinolytic activity
- α2-macroglobulin – a non-specific protease inhibitor
- thrombin-activatable fibrinolysis inhibitor (TAFI) – removes the lysine residues from fibrin, which are necessary for plasminogen and tPA binding. This makes the fibrin clot less susceptible to lysis
- plasminogen activator inhibitor-3 (PAI-3) – also known as protein C inhibitor PCI), inhibits tPA and activated protein C
- fibrinolysis is fibrin-specific
- high affinity of tPA for plasminogen in the presence of fibrin allows efficient activation of plasminogen on the fibrin barrier
- at the same time, fibrin-bound plasmin is protected against rapid inhibition by plasmin inhibitors like α2-antiplasmin); in contrast, free plasmin is rapidly inhibited
- streptokinase and urokinase are non-specific fibrinolytics and activate both circulating and fibrin-bound plasminogen, leading to widespread systemic activation of the fibrinolytic system, leading to degradation of other plasma proteins, including fibrinogen, factor V or factor VII
Components of the fibrinolytic system
| molecular weight (Da) | effect | |
| plasminogen | 88000 | proenzyme |
| plasmin | 88000 | active enzyme |
| tPA | 70000 | tissue enzyme |
| EPA | 54000 | urokinase-type |
| α2-antiplasmin | 70000 | specific fast-acting plasma inhibitor |
| PAI-1 | 43000 | endothelium-produced rapid inhibitor of both t-PA and u-PA |
- plasminogen is a zymogen (inactive precursor of an enzyme) that is synthesized primarily in the liver and circulates in the blood (plasma concentration ~ 1.5-2 pmol/L)
- there are molecular variants of plasminogen, including a type known as Lp(a), which is structurally similar to plasminogen but has a different function and is considered a risk factor for cardiovascular disease
- upon activation (via cleavage catalyzed by tPA, etc.), it is converted to plasmin, a serine protease that breaks down fibrin into soluble degradation products
- the conversion of plasminogen to plasmin is tightly regulated by inhibitors (like α2-antiplasmin and PAIs)
- disorders of the plasminogen-plasmin system can lead to either a hyperfibrinolytic state, which may result in bleeding, or a hypofibrinolytic state, which may result in thrombosis
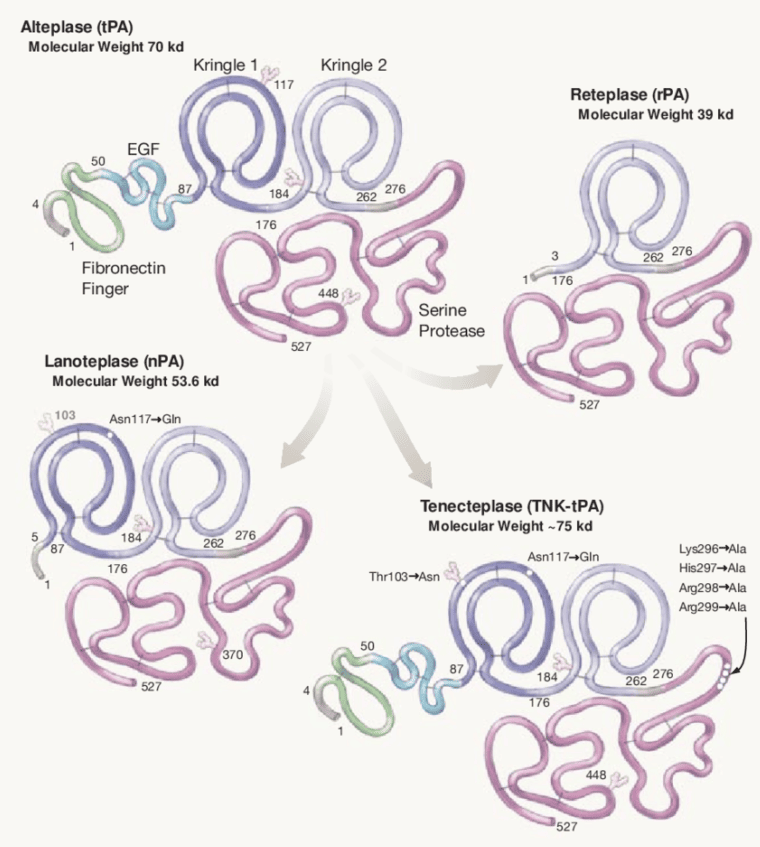
- tissue plasminogen activator (tPA) – a serine protease (alteplase, tenecteplase)
- urokinase-type activator (uPA)
- plasmin is a proteolytic enzyme that cleaves not only fibrin and fibrinogen but also factor V, factor VIII, and prothrombin
- inhibition of fibrinolysis may occur at the level of plasmin inhibition or inhibition of plasminogen activators
- α2 – antiplasmin is the main physiological inhibitor of plasmin in human plasma
- a glycoprotein belonging to the serine protease inhibitors (serpins)
- forms a complex with plasmin without protease activity
- the main inhibitor of tPA and uPA is the plasminogen activator inhibitor (PAI 1,2)
- glycoprotein belonging to serine protease inhibitors (serpins)
- is the primary inhibitor of tPA and uPA in human plasma
- reacts with tPA and urokinase but not with pro-urokinase
- plasminogen activator inhibitor-3 (PAI-3)
- also known as Protein C Inhibitor (PCI), is a serine protease inhibitor (serpin) that plays a role in various physiological processes including coagulation and fibrinolysis
- it is known to inhibit activated protein C and tissue-type plasminogen activator (t-PA), among other proteases
- it may also have roles in modulating inflammation and tumor invasion
- thrombin-activatable fibrinolysis inhibitor (TAFI)
- TAFI is activated by thrombin, thrombin in complex with thrombomodulin, or plasmin. When activated, it’s termed activated TAFI (TAFIa)
- TAFIa exerts its anti-fibrinolytic activity by removing C-terminal lysine residues from partially degraded fibrin, hence slowing down the process of fibrinolysis
| Content available only for logged-in subscribers (registration will be available soon) |
Overview of plasminogen activators (fibrinolytics)
- all fibrinolytic agents act as plasminogen activators
- alteplase and recently tenecteplase became a standard treatment for an acute ischemic stroke → intravenous thrombolysis
1st generation thrombolytics
- urokinase
- streptokinase
- no specificity for fibrin
- streptokinase activates plasminogen to plasmin indirectly – initially, streptokinase forms a complex with plasminogen, which is then converted to plasmin-streptokinase complex
- contraindicated in stroke due to a higher incidence of intracranial bleeding
2nd generation thrombolytics
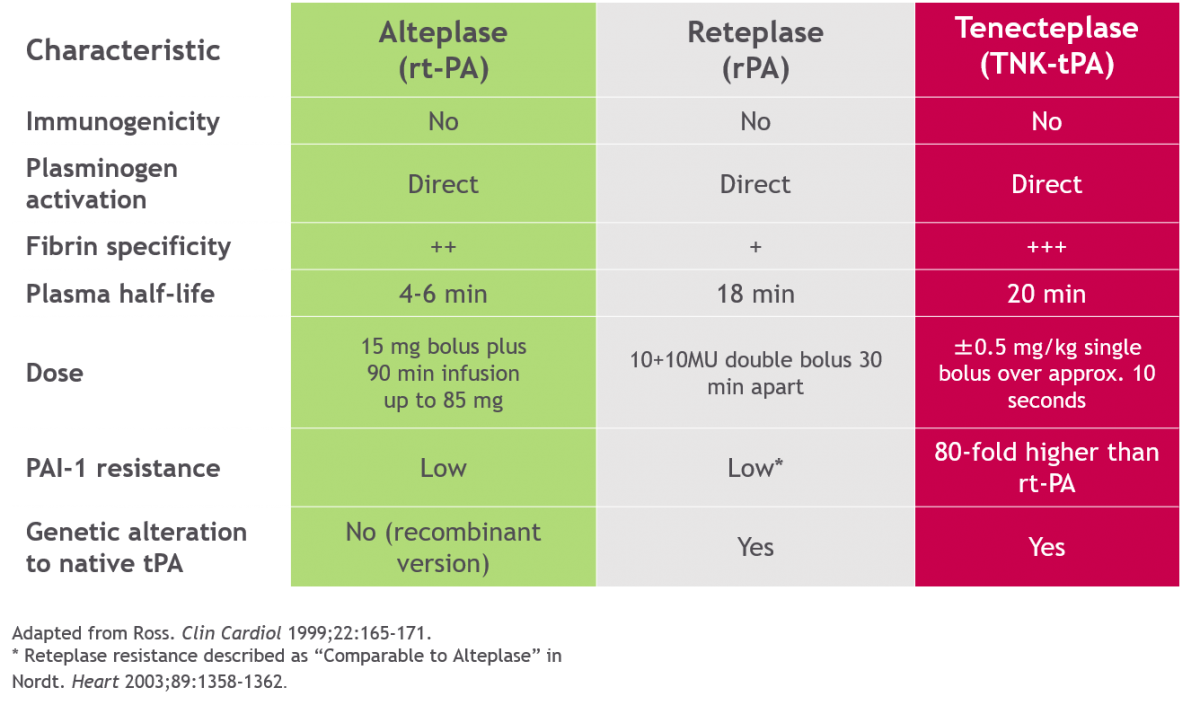
- alteplase (ACTILYSE)
- recombinant human tissue plasminogen activator (tPA)
- short half-life (3-6 minutes), administrated as a 1-hour infusion (with an initial bolus)
- the most widely used thrombolytic in stroke therapy to date → intravenous thrombolysis in acute stroke
- the high affinity of alteplase for plasminogen in the presence of fibrin allows effective activation on the fibrin barrier, whereas, in plasma, plasminogen activation by alteplase is ineffective
- theoretically, alteplase should only induce thrombolysis of the fibrin clot and not lead to a hypocoagulable state and hypofibrinogenemia; practical experiences reveal that elevated risk of bleeding and a decrease in fibrinogen levels can be observed
- theoretically, alteplase should only induce thrombolysis of the fibrin clot and not lead to a hypocoagulable state and hypofibrinogenemia; practical experiences reveal that elevated risk of bleeding and a decrease in fibrinogen levels can be observed
3rd generation thrombolytics
selective binding to fibrin, no systemic fibrinolysis, faster onset of action than generation II
- tenecteplase (METALYSE)
- a genetically engineered variant of tPA
- a longer half-life (17 ± 7 min) allows a single bolus injection
- it has greater affinity for fibrin, greater resistance to PAI
- significant stroke trials:
- EXTEND-IA TNK showed a higher incidence of reperfusion and better functional outcome with TNK compared to alteplase in patients with large artery occlusion (LVO) followed by MT (complete reperfusion 22% with TNK vs. 10% with alteplase)
- ACT (2022) and ATTEST-2 (2023) showed non-inferiority of TNK at a dose of 0.25 mg/kg compared to alteplase in a standard indication
- meta-analysis of 4 RCTs in patients with LVO [Katsanos, 2020]
- up to 4.5 hours, it can be used as an alternative to alteplase (ESO guidelines 2021)
- reteplase (RAPILYSIN / RETAVASE)
- recombinant plasminogen activator with a longer half-life than alteplase (15-18 minutes), allowing for bolus administration
- fibrinogen depletion after reteplase is less pronounced than after streptokinase but more pronounced than after alteplase
- reteplase may hold potential, but its role in stroke therapy is not yet well-established; it is used primarily in the management of acute myocardial infarction
4th generation thrombolytics
- desmoteplase
- fibrin-specific thrombolytic protein derived from the saliva of the vampire bat Desmodus rotundus
- approx. 180-fold more fibrin selective than alteplase ⇒ does not significantly affect systemic coagulation
- no effect on BBB, no neurotoxicity, and longer plasma half-life than alteplase (4 hours)
- not used in clinical practice (DEDAS, DIAS, DIAS2 trials)
Synthetic antifibrinolytics
(Exacyl / Traxyl / Transamin) usually 1ml/100mg
- slow IV injection 10mg/kg (vial=5mL/500 mg) over 10 min every 8 hours (infusion speed < 100mg/min to avoid hypotension)
- cardiac surgery – 10-20 mg/kg bolus + continuous IV infusion 1-2 mg/kg/h
- reduce dose in nephropathy
- no dosage adjustment is required for hepatic impairment
(Trasylol / Antilysin)
- vial = 10 000 TIJ (trypsin inhibitory units) / 1 mL
- a bolus of 100 000 TIJ (10 mL) IV followed by infusion of 200-300 000 TIJ (diluted in 5% glucose) within 3-4 hours
- non-specific inhibitor – interferes with the active center of serine proteases, inhibits kallikrein, trypsin, urokinase, and elastase, interferes with the contact system (f.X), inhibition of f.XII and thrombin
(Amicar)