INTRACEREBRAL HEMORRHAGE / VASCULAR MALFORMATIONS
Sturge-Weber syndrome (SWS)
Updated on 09/11/2023, published on 02/11/2023
- Sturge-Weber syndrome (SWS), also known as encephalotrigeminal angiomatosis, is a neurocutaneous disorder manifested by leptomeningeal and facial angiomas
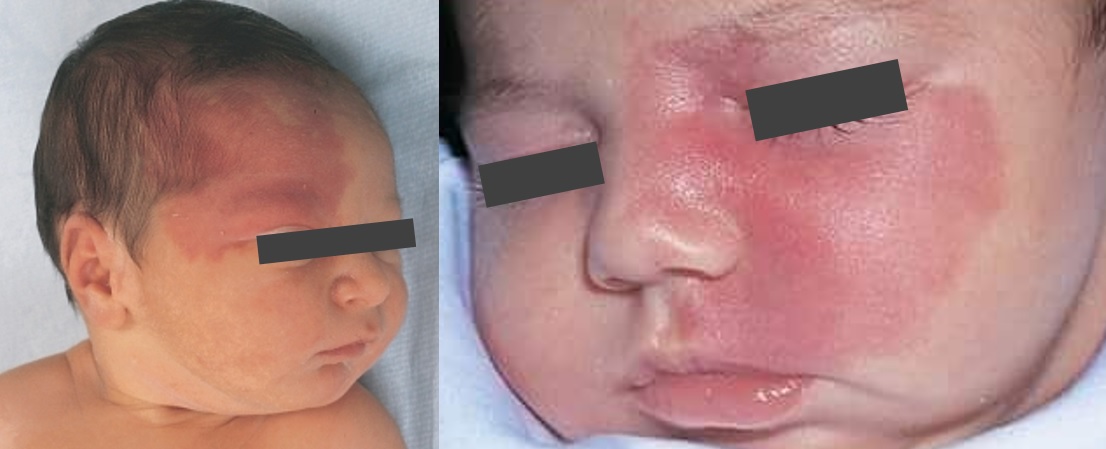
- the facial lesion, also referred to as a nevus flammeus or port-wine birthmark (PWB), is the hallmark of the disease
- it is typically located in the ophthalmic and maxillary distributions of CN V with a sharp midline demarcation
- it is caused by dilated dermal capillaries
- leptomeningeal angiomatosis results in vascular steal affecting the adjacent cortex and white matter, causing localized ischemia
- unilateral involvement occurs in ~ 80% of cases
- the facial lesion, also referred to as a nevus flammeus or port-wine birthmark (PWB), is the hallmark of the disease
- SWS is not typically characterized by stroke, but the venous malformation may predispose to venous (hemorrhagic) infarction (due to venous or sinus thrombosis)
- SWS-related stroke-like episodes may cause DDx difficulties (stroke mimics)
Etiology
- SWS is a sporadic disease not associated with hereditary transmission (unlike other neurocutaneous disorders, such as neurofibromatosis or tuberous sclerosis)
- SWS occurs due to somatic mutations that are not inherited; mutation appears to cause alterations in the regulation of the structure and function of blood vessels, vascular innervation, and expression of extracellular matrix and vasoactive molecules
- an associated gene mutation has been identified in the GNAQ gene (p.Arg183Gln) on chromosome 9 (at 9q21.2)
- development of isolated port wine stains or SWS depends on what stage of embryonic development is affected
- somatic pathogenic variants in GNAQ occurring at a later stage in embryogenesis may affect only precursors of vascular endothelial cells and lead to nonsyndromic port wine stains, while those occurring at an earlier stage affect a variety of precursor cells and cause SWS
- atypical SWS associated with variants in GNA11 has been reported
- an associated gene mutation has been identified in the GNAQ gene (p.Arg183Gln) on chromosome 9 (at 9q21.2)
Classification
SWS can be classified according to the presence/absence of facial and leptomeningeal angiomas (Roach, 1992):
- type I: classic syndrome (most common) with both facial and leptomeningeal angiomas ± glaucoma
- seizures usually occur within the first year of life
- in rare cases, the facial and brain involvement is bilateral
- mental and physical development is impaired to varying degrees
- type II: facial angioma without evidence of intracranial disease ± glaucoma
- type III: isolated leptomeningeal angioma with no facial involvement, usually without glaucoma
Clinical presentation
- a congenital facial cutaneous capillary malformation (also known as port wine stain or nevus flammeus)
- almost always present (95%)
- usually involves the V1 or V2 portion of the trigeminal nerve
- the nevus is most commonly unilateral and ipsilateral to the intracranial abnormality
- childhood epilepsy (up to 90% of cases)
- seizures usually occur in the first few years of life and are often associated with developmental delay and hemispheric symptoms, including hemiplegia/hemiparesis and hemianopsia
- often refractory to medical therapy
- ocular manifestations
- choroidal or (epi)scleral angiomatosis
- hemangioma-like superficial changes in the eyelid
- retinal detachment
- tortuous retinal vessels
- buphthalmos (enlarged eyeball due to increased intraocular pressure)
- glaucoma
- developmental delay, learning disabilities, attention deficit hyperactivity disorder
- macrocephaly
- stroke-like episodes
- weakness or paresthesias on one (or both) sides of the body
- a wide range of recovery times to baseline motor function was reported (1 minute to 6 months, median: 24 hours)
- exact etiology is unknown; it may be triggered by seizures or migraines, but it may also develop without any evidence of epilepsy
- brain MRI rarely shows permanent infarcts typical for cerebrovascular events of arterial origin
- hemorrhagic venous infarction (rare)
Factors suggesting a progressive course of cortical damage in SWS include:
- initial focal seizures progressing to frequent secondarily generalized seizures
- increasing seizure frequency and duration despite the use of antiseizure medication (ASMs)
- increasing duration of a transient postictal deficit
- increase in focal or diffuse atrophy (demonstrated by serial neuroimaging)
- progressive calcifications
- development of hemiparesis
- cognitive deterioration
Diagnostic evaluation
Neuroimaging
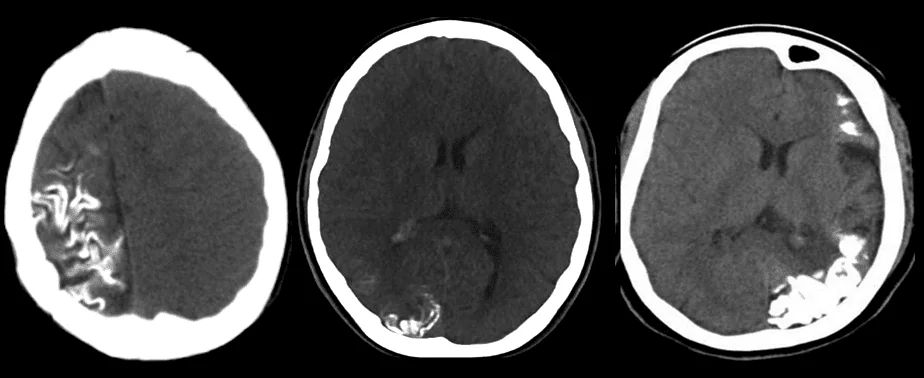
- gyriform calcifications of subcortical white matter
- CT/MRI has replaced this method
- T1: usually normal, atrophy occurs at an older age
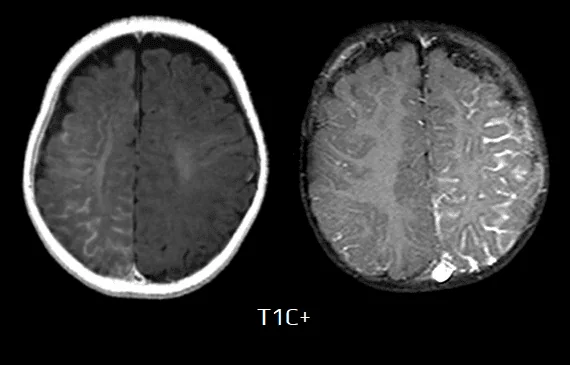
- T1 C+ (Gd)
- leptomeningeal enhancement in the affected area due to congested internal cerebral veins (pial angiomatosis) resulting in venous congestive ischemia
- later, the angioma may lose its enhancement
- enlarged ipsilateral choroid plexus
- dilatation of transparenchymal veins communicating between the superficial and deep venous systems
- T2 – low signal in white matter near to the angioma represents:
- calcifications
- abnormal deep venous drainage (with flow voids)
- GE/SWI/EPI: subcortical hypointense lesions
- MR spectroscopy: decreased NAA
- angiography demonstrates absent superficial cortical veins with abnormal and enlarged deep venous drainage
- decreased arterial blood flow velocity and increased pulsatility were found in the MCA and PCA, suggesting high resistance
- these results may reflect high venous stasis, potentially contributing to chronic hypoperfusion
Other methods
- ophthalmologic examination (glaucoma, etc.)
- cerebrospinal fluid (CSF) examiantion
- protein may be elevated, presumably secondary to microhemorrhage
- electroencephalography (EEG)
Differential diagnosis
DDx of intracranial calcifications + cerebral hemiatrophy + leptomeningeal enhancement
- cerebral arteriovenous malformation (AVM)
- infection
- TORCH infection (vertically transmitted infections that may cause severe fetal anomalies or fetal loss)
- Toxoplasmosis
- Other infections (e.g., Varicella-Zoster Virus, HIV, Syphilis)
- Rubella
- Cytomegalovirus (CMV)
- Herpes Simplex Virus (HSV)
- neurocysticercosis
- TORCH infection (vertically transmitted infections that may cause severe fetal anomalies or fetal loss)
- PHACE syndrome
- also known as cutaneous hemangioma–vascular complex syndrome or Pascual-Castroviejo type II syndrome
- radiation-induced injury
- Gobbi syndrome
- combination of celiac disease, epilepsy, and bioccipital calcifications
- calcification secondary to intrathecal methotrexate therapy and meningitis
- Dyke-Davidoff-Masson syndrome
- one cerebral hemisphere is partially or completely atrophic as a result of an intrauterine or perinatal carotid artery infarction
Management
- seizure control (ASM ± surgery)
- symptomatic and prophylactic treatment of headaches
- glaucoma treatment
- laser therapy for skin lesions