ISCHEMIC STROKE / PREVENTION
Management of intracranial stenosis
Updated on 24/07/2024, published on 10/12/2023
- intracranial stenosis is a major factor that increases the risk of stroke [Bos, 2014]
- it is the cause of ~10% of strokes
- the annual risk of recurrent stroke in symptomatic IC stenosis is 4-18%
- EC/IC Bypass Trial, WASID
- the highest risk is present in the first 14 days after the initial event
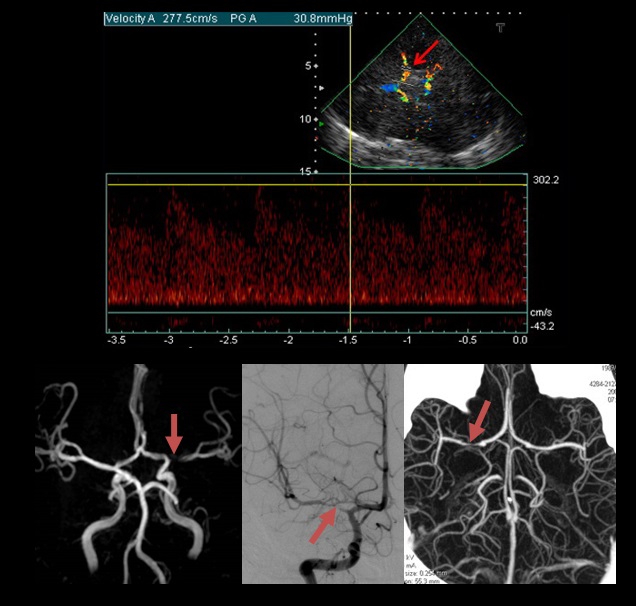
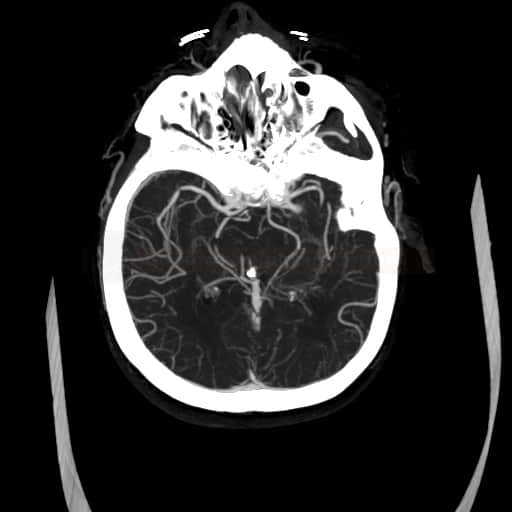
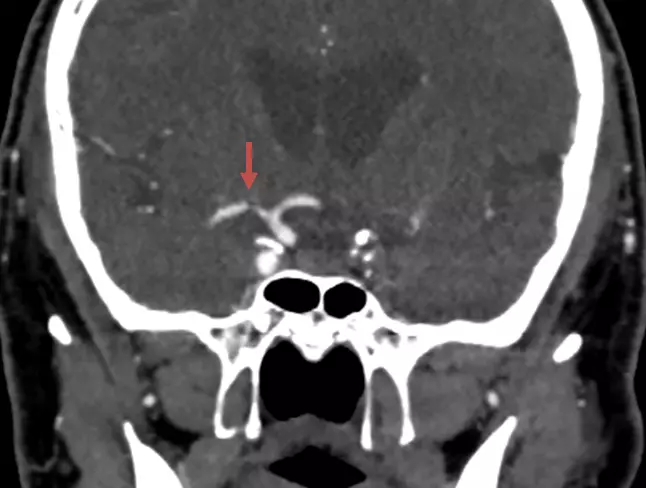
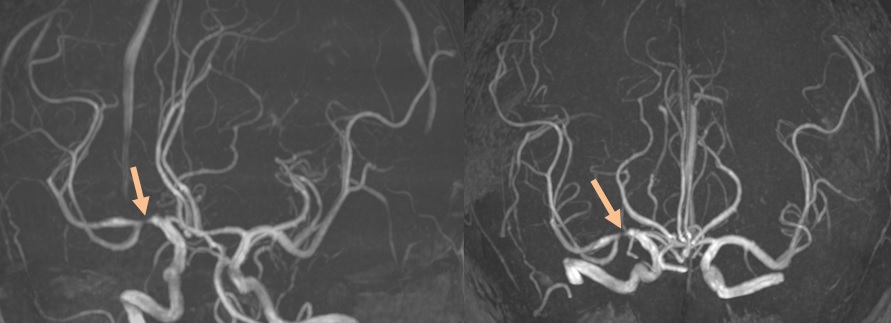
- modern imaging modalities (TCD/TCCD, CTA, and MRA) allow non-invasive and easy diagnosis of intracranial stenosis [Degnan, 2011]
Etiopathogenesis
| Mechanisms of stroke in IC stenosis |
|
|
| The most common causes of intracranial stenosis | |
|
- progression of stenosis can occur for the following reasons:
- growth of atherosclerotic plaque
- thrombosis
- plaque rupture with intraplaque hemorrhage
TCD/TCCD
-
detection of the stenosis itself (→ see here)
- optimal for screening and follow-up
-
evaluation of the distal flow and cerebral vasomotor reactivity (CVR)
-
detection of MES (microembolic signals)
CT/MR angiography
-
the optimal imaging method is CTA
-
non-contrast intracranial MRA may overestimate the stenosis grade
Digital subtractive angiography
- currently replaced by CTA in most cases
Xe133-CT
- enables to determine the cerebral blood flow (CBF)
Intravascular sonography (IVUS) [Zacharatos, 2010]
- used in cardiology, now being tested in carotid and intracranial territories
- clarifies the morphology of the stenosis (atherosclerotic plaque vs. inflammation, etc.)
Optic coherent tomography (OCT)
- OCT uses near-infrared light to image the arterial anatomy with much higher resolution than IVUS (10 vs. 100 μm)
- used in cardiology, also tested in vascular neurology
- assessment of plaque structure (Yang, 2021)
- assessment of stent position after the procedure and at follow-up
- potential for use during the stenting procedure is investigated
- assessment of plaque structure (Yang, 2021)
- outperforms IVUS in determining plaque morphology (presence of thrombus, hemorrhage, fibrous cap rupture, etc.) and in differentiating other causes of stenosis
Management
| Asymptomatic stenosis |
- antiplatelet therapy + aggressive treatment of vascular risk factors (see below)
- endovascular intervention is not indicated
| Symptomatic stenosis |
- the risk of recurrent stroke/TIA or vascular death in patients with IC stenosis is 10-12%/year [Chimowitz,2005]
- older studies reported a risk of up to 22%/year with conservative treatment; despite that, the risk is ~2 times higher than in patients with extracranial stenosis
- the mainstay of treatment of intracranial and extracranial stenoses is aggressive treatment of vascular risk factors
Aggressive treatment of vascular risk factors
- target BP < 120/80 mm Hg (if possible with respect to comorbidities) AHA/ASA 2021 1/B-NR)
- careful with lower values, hypoperfusion behind severe stenosis may be provoked
- in therapy, prefer ACE-I or sartans
- intensive lipid-lowering therapy with target LDL < 1.4 mmol/l (after stroke/TIA) → target lipid values
- SPARCL trial showed a reduction in the recurrence risk in patients with recent stroke/TIA
- tight glycemic control
- achieve target glycosylated hemoglobin + manage insulin resistance
- patients with diabetes < 6% (60 mmol/mol)
- patients without diabetes < 4.5% (45 mmol/mol)
- lifestyle interventions:
- smoking cessation → see here
- obesity control
- according to some recommendations, the target BMI <20-24!
- waist circumference female/ male <80/<94 cm
- healthy diet (e.g., Med-Diet)
- appropriate and regular physical activity
- 150 min/wk of moderate aerobic activity or 75 min/wk of vigorous aerobic activity
Anticoagulant vs. antiplatelet therapy
- the mainstay of medical treatment is antiplatelet therapy (AHA/ASA 2021 I/B-R)
- WASID trial did not show the superiority of anticoagulants over antiplatelet therapy
- start ASA 100-325 mg/d
- efficacy of clopidogrel, cilostazol, or ticagrelor in monotherapy is unknown (AHA/ASA 2021 2b/C-EO)
- WASID trial did not show the superiority of anticoagulants over antiplatelet therapy
- in patients with recent stroke/TIA (<30 days) and stenosis >70%, DAPT (ASA+CLP 75mg) for up to 90 days may be considered – SAMMPRIS (AHA/ASA 2021 2a/B-NR)
- in patients with recent minor stroke/TIA (< 24h) and intracranial stenosis > 30%, DAPT with ASA + ticagrelor 2x 90mg for 30 days may be considered (AHA/ASA 2021 2a/B-NR)
- DAPT ASA/CLP + cilostazol 200 mg/d may be considered in patients with recent stroke/TIA (< 24h) and intracranial stenosis > 50% (AHA/ASA 2021 2b/B-NR)
- anticoagulation may be considered when antiplatelet therapy is ineffective or in cases of stenoses with extensive thrombosis
Percutaneous transluminal angioplasty with stenting (PTAS)
- due to the small diameter of the intracranial arteries, the risk of complications during the endovascular procedures is higher than for procedures on the extracranial arteries
- significant risk of vasospasm, dissection, and arterial rupture with high mortality and morbidity
- stenting may be difficult if there is extracranial kinking
- on the other hand, even mild dilatation of an intracranial stenosis may be beneficial as it leads to a significant improvement in blood flow
- hemodynamic failure is an important mechanism for the development of ischemia in intracranial stenoses (as opposed to extracranial stenoses where distal embolization prevails)
- the SSYLVIA and ASS1T-1 trials have demonstrated the technical feasibility of intracranial angioplasty
- successful stent deployment was achieved in 95-97.5% of cases, with restenosis >50% in up to 30% of patients/year
- however, the SAMMPRIS and VISSIT (Vitesse Intracranial Stent Study for Ischemic Stroke Therapy) trials failed to show the benefit of stenting over medical therapy
- for symptomatic stenoses of 50-69%, the procedure is associated with high mortality and morbidity compared to medical therapy (AHA/ASA 2021 3/B-NR)
- for symptomatic stenoses of 70-99%, angioplasty should not be the first-line therapy, even in patients already on monotherapy (AHA/ASA 2021 3/A) (⇒ switching to DAPT is preferred)
- angioplasty may be considered as the last option when aggressive medical therapy fails in stenoses of 70-99% – however, the benefit is unknown (AHA/ASA 2021 2b/C-LD)
- stenosis > 70% + failure of maximal medical therapy
- the SAMMPRIS trial evaluated the efficacy and safety of percutaneous transluminal angioplasty and stenting (PTAS) with the Wingspan system in symptomatic severe atherosclerotic intracranial stenosis (70-99%) compared to aggressive medical therapy alone
- 30-day stroke/death 14.7% vs 5.8% (conservative treatment)
- primary endpoint at 1 year: 12% (aggressive conservative treatment) vs. 20% (PTAS)
- patients did not benefit from endovascular therapy because of a high risk of periprocedural complications and a low risk of stroke recurrence with aggressive conservative treatment
- the high efficacy of aggressive conservative treatment supports the fact that in SAMMPRIS, patients treated conservatively were less likely to have a recurrent stroke than in the WASID trial
- the superiority of conservative management persisted at 2-4 year follow-up (14.1% vs. 20.6% at 2 years (P = 0.07) and 14.9% vs. 23.9% at 3 years (P =0 .0193) (Derdeyn, 2013)
- negative results were also reported in the VISSIT trial (Vitesse Intracranial Stent Study for Ischemic Stroke Therapy) [Zaidat, 2015]
- using a balloon-expandable stent compared with medical therapy alone led to a higher risk of stroke/TIA in the same territory after 12 months and a higher risk of any stroke or TIA within 30 days
Surgery (EDAS – Encephalo-Duro-Arterio-Synangiosis)
- positive results of the phase 2a ERSIAS trial (annual risk of recurrence 9.6% vs. 21.2%; randomized trial is planned)
EC-IC bypass
- not recommended for intracranial stenoses/occlusions (AHA/ASA 2021 3/B-R)
Intracranial angioplasty procedure
- continue dual antiplatelet therapy (ASPIRIN 100 mg + CLOPIDOGREL 75 mg) after the procedure
- the duration of DAPT depends on the type of stent:
- coated stents (such as Taxus or Pharos): 6-12 months
- standard stent (such as Wingspan): ≥ 4 weeks
- after the required duration of DAPT, switch to long-term single antiplatelet therapy (SAPT)