ADD-ONS / MEDICATION / ANTICOAGULATION
Andexanet alfa
Updated on 17/06/2024, published on 25/01/2022
- the introduction of direct oral anticoagulants (DOACs) revolutionized anticoagulation therapy
- however, FXa inhibitors, in particular, present a challenge in the management of acute bleeding episodes
- until recently, prothrombin complex concentrates (PCC) have been used off-label to treat acute, life-threatening bleeding secondary to factor Xa (FXa) inhibitors
- andexanet alfa (ONDEXXYA, ANDEXXA), a recombinant modified human factor Xa molecule, was developed as a targeted reversal agent for these anticoagulants
- andexanet was approved by the FDA in 2018 for the reversal of life-threatening bleeding in patients anticoagulated with FXa inhibitors → more here
Mechanism of action
- the drug is a modified recombinant inactive form of human FXa that specifically binds to and sequesters FXa inhibitor molecules, thereby rapidly reducing their activity
- positive trials supporting its use include ANNEXA-A, ANNEXA-R, and ANNEXA-4 [Medscape 2016]
- it is administered as an IV bolus over 15-30 minutes, followed by a 2-hour infusion
- guidelines now recommend the use of andexanet alfa as the first-line therapy
- ANNEXA-4 was a multicenter, prospective, open-label, single-arm trial evaluating patients experiencing major bleeding
- the trial involved 352 participants
- all patients received a bolus of andexanet alfa, followed by a 2-hour infusion to treat acute major bleeding after taking a factor Xa inhibitor in the previous 18 hours
- ICH 64%, GIT bleeding 26%
- rivaroxaban 36%, apixaban 55%, edoxaban 3%, enoxaparin 6% (those with doses > 1 mg/kg)
- the study evaluated the decrease in anti-Xa activity and hemostatic efficacy 12 hours post-infusion
- apixaban and rivaroxaban showed a 92% decrease in mean anti-Xa activity; enoxaparin showed a 75% decrease
- hemostasis at 12 hours was rated as excellent or good in 82% of patients
- the 30-day mortality was 14%, with thrombotic events occurring in 10% of patients, and two patients had mild post-infusion reaction
Andexanet and intracerebral hemorrhage (ICH)
- ANNEXA-I (2024) trial showed mixed effects of andexanet in patients with ICH who are taking factor Xa inhibitor anticoagulants
- andexanet resulted in better control of hematoma expansion than standard care (where PCC was used in 85%) but was associated with an increased risk of thrombotic events, including ischemic stroke
- hematoma expansion ≥12.5 mL – andexanet 11.1% (24/216) vs. standard care 16.8% (36/214)
- the anti-FXa activity was significantly reduced in patients with ICH treated with andexanet alfa
- thrombotic events occurred in 10.3% vs 5.6% (standard care), ischemic stroke occurred in 6.5% vs. 1.5% (standard care)
- there were no appreciable differences between the groups in the score on the modified Rankin scale or in death within 30 days (traditional 90-day mRS is not available)
Dosing
Standard dose
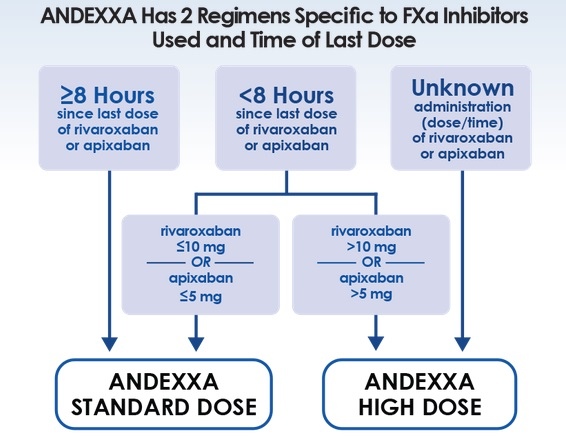
- ≥ 8 hours after DOAC administration
- < 8h after administration of rivaroxaban ≤ 10 mg or apixaban ≤ 5 mg
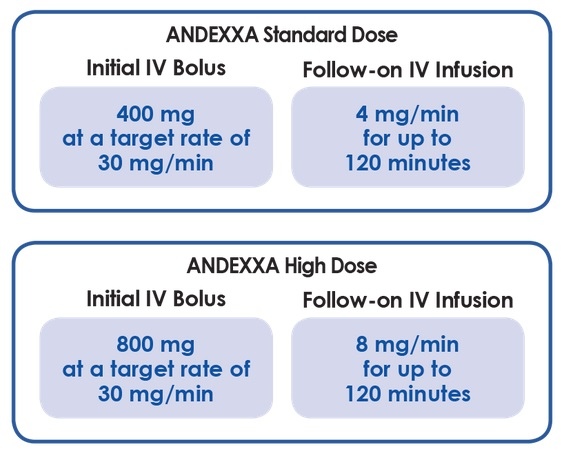
- administer IV bolus of 400mg at a rate of 30 mg/min (~ 13 min)
- followed by an infusion at a rate of 4 mg/min for 120 min (total dose = 480 mg)
Higher dose
- use if the dose or time of the last DOAC administration is uncertain or if < 8h have passed since administration of > 10 mg rivaroxaban or > 5 mg apixaban
- give an IV bolus of 800mg at a rate of 30 mg/min (approximately 13 min)
- followed by an infusion at a rate of 8 mg/min for 120 min (total dose = 960 mg)
FAQs
- andexanet alfa is a recombinant, inactive form of Factor Xa
- it is used to reverse life-threatening bleeding in patients who are being treated with certain factor Xa inhibitors, such as apixaban and rivaroxaban
- andexanet alfa acts by binding to and sequestering factor Xa inhibitors in the bloodstream. This action neutralizes their anticoagulant effects, thereby helping to control severe bleeding events
- it is specifically used in emergencies to reverse life-threatening bleeding in patients treated with factor Xa inhibitors, especially when the bleeding is intracranial or gastrointestinal
- yes, the efficacy of andexanet alfa has been demonstrated in clinical trials, notably the ANNEXA-4 trial, which evaluated its effectiveness and safety in stopping major bleeding events
- some potential side effects include thromboembolic events, such as heart attack or stroke, and infusion-related reactions
- it’s important to monitor patients closely during and after its administration
- andexanet alfa is administered intravenously
- the dosage depends on the specific factor Xa inhibitor, its dose, and the time since its last dose
- andexanet alfa is primarily effective for reversing the effects of apixaban and rivaroxaban
- its effectiveness with other factor Xa inhibitors, like edoxaban and enoxaparin, may vary
- the safety of andexanet alfa in pregnant or breastfeeding women has not been established
- its use should be considered only if the potential benefit justifies the potential risk to the fetus or infant