INTRACEREBRAL HEMORRHAGE
Diagnosis of intracerebral hemorrhage
Updated on 17/06/2024, published on 27/11/2023
- for patients presenting with stroke-like symptoms, rapid neuroimaging using CT or MRI is recommended to confirm the diagnosis of spontaneous intracerebral hemorrhage (ICH)
- the detection of intracerebral bleeding itself by imaging methods is easy; the diagnostic evaluation is focused on determining the underlying etiology
Physical Examination and focused history
- focused medical history (see tab below)
- quick physical examination → Early management of patients with suspected stroke
- vital signs – assess airway, breathing, circulation
- a general physical examination should focus on the head, heart, lungs, abdomen, and extremities
- a focused neurological examination + NIHSS
- assess GCS in patients with impaired level of consciousness (LOC)
- time of symptoms onset (or time patient was last seen normal)
- symptoms
- headache
- thunderclap: aneurysm, RCVS, rarely CVST
- slower onset: mass lesion, CVST, ischemic stroke with hemorrhagic transformation
- focal neurologic deficits
- seizures
- decreased level of consciousness
- headache
- vascular risk factors
- prior ICH or SAH
- hypertension
- hyperlipidemia
- diabetes
- metabolic syndrome
- imaging biomarkers (e.g., cerebral microbleeds)
- medications
- anticoagulant drugs
- thrombolytic drugs
- antiplatelet agents
- NSAIDs
- vasoconstrictors (associated with RCVS): triptans, SSRIs, decongestants, stimulants, phentermine, sympathomimetics
- antihypertensives (as a marker of chronic hypertension)
- estrogen-containing oral contraceptives (↑risk of CVST)
- cognitive impairment or dementia (possible amyloid angiopathy)
- substance use
- smoking
- alcohol use
- marijuana (associated with RCVS)
- sympathomimetic drugs (amphetamines, methamphetamines, cocaine)
- liver disease, uremia, malignancy, and hematologic disorders (→ secondary coagulopathy)
Computed tomography (CT)
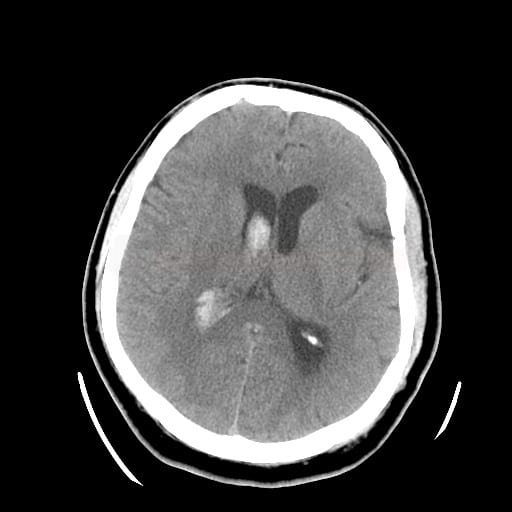
Non-contrast CT scan (NCCT)
- baseline examination objectives:
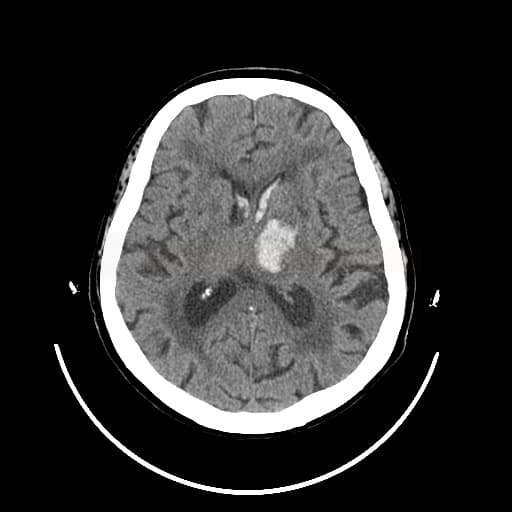
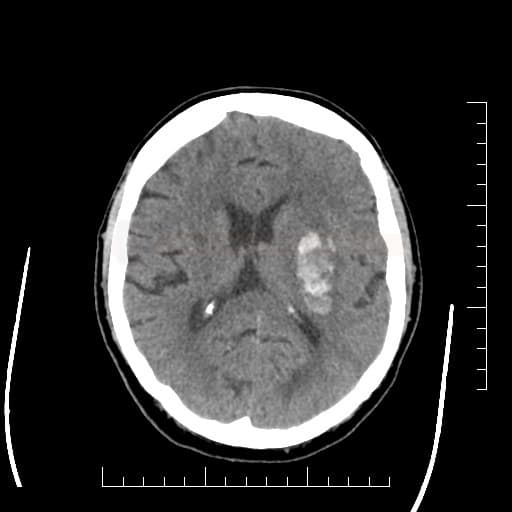
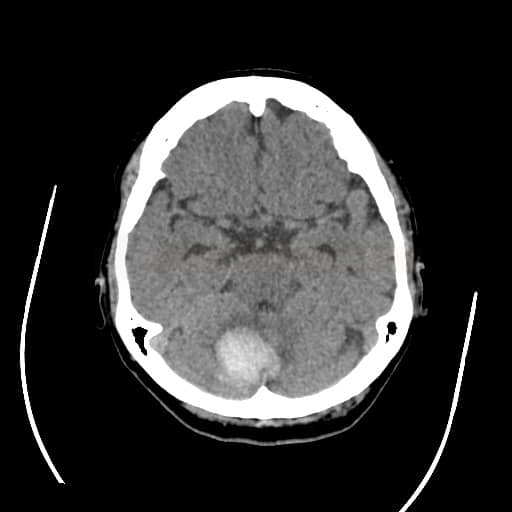
- detect and localize the hematoma
- assess its type, volume, probable etiology, risk of complications
- assess the prognosis (→ ICH scales)
- fresh blood is hyperdense on NCCT
- increased density is caused by the high hemoglobin content of retracted clot or sedimented blood
- the density of the hematoma in the acute stage is typically around 70-80HU; if the lesion has a density > 100-120HU, , it is likely to be calcification, foreign body, etc.
- resorption of the hematoma occurs within days to weeks; accompanied by a decrease in its density
- hematoma becomes isodense within 1-6 weeks
- in the chronic stage, a hypodense pseudocyst with atrophy of the surrounding brain tissue is usually found at the site of the resorbed hematoma (indistinguishable from old ischemia)
- increased density is caused by the high hemoglobin content of retracted clot or sedimented blood
- vasogenic edema gradually appears around the subacute hematoma as a hypodense area
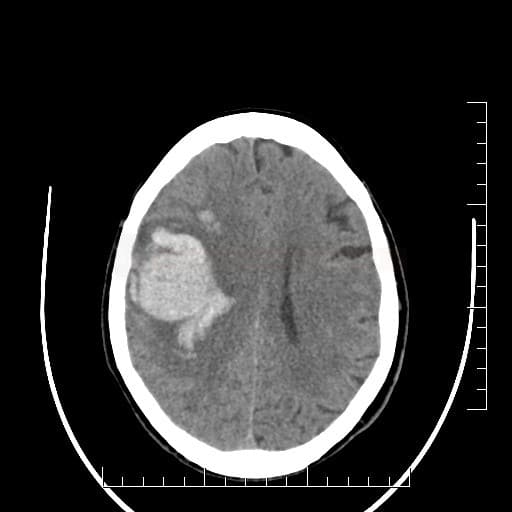
- type and location of bleeding:
- typical “hypertensive” bleeding
- atypical bleeding → search for structural cause (CTA/MRI+MRA/DSA)
- lobar hematomas
- primary intraventricular hemorrhage (IVH) – exclude AVM or small aneurysm in the ventricular wall or SAH from AComA with perforation of the terminal lamina and bleeding into the third ventricle
- age < 50 years in the absence of hypertension despite typical ICH location
- large and early vasogenic edema
- lobar hematomas
- typical “hypertensive” bleeding
- there are several NCCT predictors of hematoma expansion and unfavorable outcome in acute ICH
- Satellite sign, Island sign – subtle hemorrhages near the main hematoma
[Li, 2017] [Shimoda, 2017]
- Blend sign
[Li, 2017]
- Black Hole sign
[Li, 2016]
- Satellite sign, Island sign – subtle hemorrhages near the main hematoma
| Content available only for logged-in subscribers (registration will be available soon) |
| Content available only for logged-in subscribers (registration will be available soon) |
CT angiography (CTA)
- CTA (+/- venography) is useful for the identification of the potential source of bleeding (vascular malformations) or cerebral venous thrombosis
- it can be performed as part of the baseline CT scan in patients with:
- age < 50 years
- atypical hematoma location/appearance
- hematoma in typical location without history of hypertension
- isolated intraventricular hemorrhage
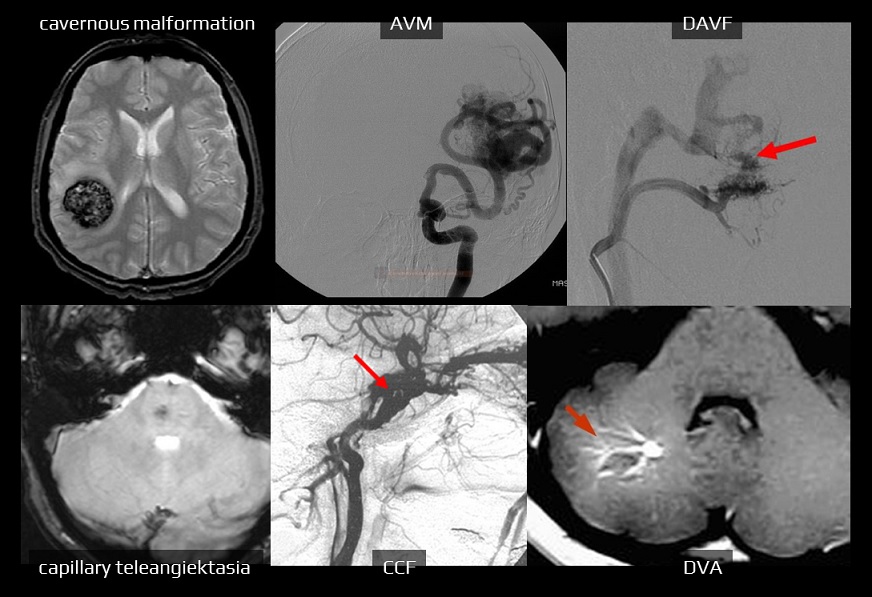
- in atypical parenchymal hematomas, add MRI+MRA or DSA if baseline CTA is negative
- it may be reasonable to start with MRI and MRA to establish a non-macrovascular cause of ICH (such as CAA, deep perforating vasculopathy, cavernous malformation, or malignancy)
- MRI may help to rule out the hemorrhagic transformation of ischemia
- search for markers of hemorrhage expansion
- spot sign on source CTA images or post-contrast CT images
- prognostically unfavorable sign of ongoing bleeding and hematoma growth (Havsteen, 2014] [Sorimachi, 2013)
- accuracy of spot sign detection is high (87%); studies with hemostatic drugs in patients with positive spot sign are planned (STOP-IT, SPOTLIGHT, STOP-AUST) [Huynh, 2013]
- “leakage sign” may also serve as a predictor of continued bleeding
[Orito, 2016]
- initial CTA + delayed phase (5 min interval)
- a 10% increase in HU density indicates ongoing bleeding
- spot sign on source CTA images or post-contrast CT images
Magnetic resonance imaging (MRI)
- MRI of the brain is highly sensitive for detecting intracerebral hemorrhage
- the appearance of hemorrhage depends on the stage of hemoglobin breakdown (see table below)
- oxy-Hb is weakly diamagnetic, deoxy-Hb has 4 unpaired electrons per iron atom and is strongly paramagnetic
- gradient recalled echo (GRE) can detect early bleeding with similar sensitivity to NCCT ⇒ MRI can be used as the initial imaging method in stroke programs (Kidwell, 2004) → see here
- hyperacute hematoma:
- T1 isointense
- T2/FLAIR – isointense or slightly hyperintense
- GRE hypointense (initially only hypointense rim + core of heterogenous signal intensity due to the diamagnetic oxyhemoglobin
- DWI hyperintense, ADC hypointense
- hyperacute hematoma:
| Content available only for logged-in subscribers (registration will be available soon) |
Digital subtraction angiography (DSA)
- indicated in search for the source of bleeding (consider patients’ age, clinical condition, comorbidities, prognosis, the location and extent of bleeding)
- DSA is often replaced by CTA/MRA, which can be performed quickly and without significant risk as part of the initial diagnostic workup
- DSA should be performed if CTA/MRA suggests a macrovascular cause of bleeding (AHA/ASA guidelines 2022, 1/C-LD)
- DSA confirms the diagnosis of a malformation and provides additional information about the main tributaries, presence, type, and extent of the nidus, type of flow (high or low flow), venous drainage, and other features (stenosis of a draining vein, intranidal or perinidal aneurysm, etc.)
- DSA is performed when CTA/MRA is negative or equivocal, and there is a reasonable suspicion of a bleeding source
- age < 50 years in the absence of hypertension
- atypical location or appearance of the hematoma
- typical location with no history of hypertension
- isolated intraventricular hemorrhage (AHA/ASA guidelines 2022, 1/BNR)
- in patients with spontaneous ICH and a negative DSA and no clear microvascular diagnosis or other structural lesions, it may be reasonable to repeat DSA 3-6 months after ICH to identify a previously obscured vascular lesion
Other examinations and tests
Blood tests
- complete blood count (check platelets)
- admission anemia is associated with hemorrhagic expansion and poor outcome
- thrombocytopenia is associated with increased mortality
- coagulation tests (to exclude anticoagulant-related hemorrhage and coagulopathy from other causes)
- APTT, Quick, INR, TT, fibrinogen (especially after thrombolysis)
- ECT and Hemoclot (dabigatran)
- specific anti-Xa (xabans)
- glucose
- admission hyperglycemia is associated with unfavorable short- and long-term outcome
- urea nitrogen, osmolality
- liver tests (coagulopathy secondary to liver failure)
- ionogram including Ca2+, Mg2+
- creatinine/estimated glomerular filtration rate (GFR)
- renal failure on admission is associated with poor functional outcome
- possible altered clearance of DOACs
- D-dimers
- cardiac enzymes + troponin
- inflammatory markers (ESR, CRP) – infective endocarditis?
- urine toxicology screen (sympathomimetic drugs are associated with ICH)
- pregnancy test in a woman of childbearing potential (exclude peripartum angiopathy, eclampsia, HELLP syndrome, and cerebral sinus thrombosis)
Others
- 12-lead ECG on admission, followed by continuous ECG monitoring
- pulse oximetry
- blood pressure monitoring (invasive/noninvasive)
- chest x-ray