ADD-ONS / MEDICATION / ANTICOAGULANTS
Low Molecular Weight Heparins ( LMWHs )
Updated on 02/12/2023, published on 18/11/2021
- Low Molecular Weight Heparins (LMWHs) are anticoagulant drugs derived from Unfractionated Heparin (UFH) by chemical or enzymatic depolymerization, transforming longer heparin chains into shorter ones. These shorter strands give LMWH a longer duration and more predictable action compared to UFH
- LMWHs have reduced inhibitory activity against thrombin relative to factor Xa
- LMWHs are used in the treatment or prophylaxis of venous thromboembolic disease (VTE)
- VTE = deep vein thrombosis (DVT) ± pulmonary embolism (PE)
- the mechanism of action, adverse event profile, pharmacology, monitoring, and relevant interactions of LMWH are discussed
Mechanism of action
- LMWHs inhibit the final common pathway of the coagulation cascade (conversion of fibrinogen into fibrin)
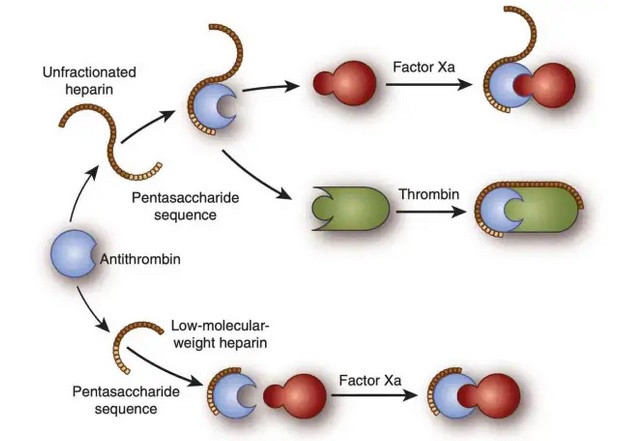
- anti-Xa and, to a lesser extent, anti-IIa effects of LMWH are mediated by antithrombin III (heparins are indirect thrombin inhibitors) (Gerotziafas, 2007)
- AT III binds to and inhibits factor Xa and factor IIa ⇒ prothrombin is not activated to thrombin, preventing the conversion of fibrinogen to fibrin for clot formation
- LMWH acts as a cofactor, increasing the inhibitory activity of AT III on clotting proteases by up to 2000-fold
- heparin inhibits both Xa and thrombin (while LMWH primarily targets Xa inhibition)
- only pentasaccharide chains with at least 18 saccharide units long can inactivate thrombin (IIa)
- AT III binds to and inhibits factor Xa and factor IIa ⇒ prothrombin is not activated to thrombin, preventing the conversion of fibrinogen to fibrin for clot formation
- the anti-Xa activity of individual LMWHs is not directly correlated with the anticoagulant and antithrombotic effect, because each LMWH has several effects mediated by mechanisms other than the inhibition of the Xa effect (such as inhibition of leukocyte procoagulant activity, antiplatelet effect, promotion of fibrinolysis, restitution of endothelial dysfunction, or ↑ TFPI)
- their ability to inactivate thrombin already bound by fibrin is limited
- LMWH does not inhibit platelet function
| Pharmacokinetic characteristics | Clinical significance |
| reduced protein binding | good bioavailability predictable dose-response no resistance observed |
| predictable dose-response | dosage according to body weight, no monitoring required |
| higher plasma half-life | dosage 1-2 × daily |
| low molecular weight | good absorption after subcutaneous application |
| limited effect on platelets and endothelial cells | ↓ risk of thrombocytopenia |
| absence of a specific antidote | inability to stop treatment quickly |
| limited ability to inactivate bound thrombin | incomplete dissolution of the thrombus |
| LMWH | Average molecular weight | Ratio anti-Xa/anti-IIa activity |
|---|---|---|
| Bemiparin (IVOR, ZIBOR) |
3600 | 8.0 |
| Nadroparin (FRAXIPARINE, FRAXODI) |
4300 | 3.3 |
| Reviparin | 4400 | 4.2 |
| Enoxaparin (CLEXANE, LOVENOX) |
4500 | 3.9 |
| Parnaparin | 5000 | 2.3 |
| Certoparin (SANDOPARIN, EMBOLEX) |
5400 | 2.4 |
| Dalteparin (FRAGMIN) |
5000 | 2.5 |
| Tinzaparin (INNOHEP) |
6500 | 1.6 |
| FONDAPARINUX |
1725 |
only anti-Xa |
| UFH |
15000 |
1 |
Pharmacokinetics
- LMWHs exhibit pharmacokinetic advantages over unfractionated heparin
- following subcutaneous injection, the bioavailability of LMWHs is about 90% and LMWHs yield a more predictable anticoagulant response compared to heparin
- the elimination half-life of LMWHs, ranging from 3 to 6 hours after subcutaneous administration, is dose-independent, and anti-Xa levels peak 3-5 hours after dosing
- a notable limitation of LMWHs is that their predominant renal clearance extending their biological half-life in patients with renal impairment
Indications
- prevention of venous thromboembolic disease (VTE) in intermediate- and high-risk populations (surgical, orthopedic, and medical patients)
- treatment of deep vein thromboses (DVT) and pulmonary embolism (PE)
- treatment of cerebral venous sinus thrombosis (CVST)
- bridging therapy during the interruption of warfarin therapy or when the INR is not within a therapeutic range
- treatment of STEMI
- LMWHs do not cross the placenta and do not harm the fetus ⇒ preferred anticoagulants in pregnancy
- UHF, LMWHs, and fondaparinux are compatible with breastfeeding
- LMWHs are also the preferred treatment for cancer-related thromboembolic disease
LMWH Dosing
| Medication |
Prophylactic dose
|
Therapeutic dose (full anticoagulation)
|
|
FRAXIPARINE
nadroparin
1ml/9500IU
|
0.3 ml (2850IU) s.c. 1-2 x daily
|
100 IU/kg 2x daily
(70kg= 2 x 0,7 ml)
|
|
CLEXANE
enoxaparin
1ml/10000IU/100mg
|
0.4 ml s.c. 1x daily (high-risk)
0.2 ml s.c. 1x daily (low-risk)
|
100 UI (1mg) /kg 2x daily
(70kg=2 x 0,7 ml )
|
Intermediate dose:
- enoxaparin 0.5 mg-1 mg/kg administered SC once or twice daily
- intermediate dosing of nadroparin is not standardized
|
Weight (kg)
|
Therapeutic dose
FRAXIPARINE (1mL/9500IU)
2x daily s.c.
|
Therapeutic dose
CLEXANE 1mL/10000IU/100mg 2x daily s.c.
|
|
<50
|
0,4 mL (3800 IU anti-Xa)
|
0,4 mL (4000 IU anti-Xa)
|
|
50-59
|
0,5 mL (4750 IU anti-Xa)
|
0,5 mL (5000 IU anti-Xa)
|
|
60-69
|
0,6 mL (5700 IU anti-Xa)
|
0,6 mL (6000 IU anti-Xa)
|
|
70-79
|
0,7 mL (6650 IU anti-Xa)
|
0,7 mL (7000 IU anti-Xa)
|
|
80-89
|
0,8 mL (7600 IU anti-Xa)
|
0,8 mL (8000 IU anti-Xa)
|
|
90-99
|
0,9 mL (8550 IU anti-Xa)
|
0,9 mL (9000 IU anti-Xa)
|
|
>100
|
1,0 mL (9500 IU anti-Xa)
|
1,0 mL (10000 IU anti-Xa)
|
Renal function-based dose reduction
- there is a higher exposure to LMWH in renal insufficiency ⇒ ↑ risk of bleeding
|
Fraxiparine
|
|
|
creatinine clearance (CrCl) ≥ 0.83 ml/s
|
no dose reduction
|
|
CrCl 0.5-0.83 ml/s
CrCl < 0.5 ml/s
|
↓ dose by 25-33 %
|
|
Clexane
|
|
|
creatinine clearance (CrCl) ≥ 0.83 ml/s
|
no dose reduction
|
|
CrCl 0.5-0.83 ml/s
|
no dose reduction
|
|
CrCl < 0.5 ml/s
|
↓ dose by 50%
100 UI/kg 1x daily in the therapeutic indication
2000 IU (0,2ml) 1x daily s.c. in prophylaxis
|
| Although dosage adjustments are not necessary for enoxaparin in patients with moderate (creatinine clearance 30-50 ml/min) and mild (creatinine clearance 50-80 ml/min) renal impairment, careful clinical and laboratory monitoring is advisable |
|
Administration
- LMWHs are administered via subcutaneous (SC) injection
- compared to heparin, LMWHs have a longer half-life and more predictable action, and they are typically administered once or twice daily
LMWH bridging
- bridging anticoagulation involves the administration of a short-acting anticoagulant (usually LMWH) in the following situations:
- initiation of anticoagulant therapy
- periprocedural bridging → see separate chapter
Monitoring of the anticoagulant effect
- check platelets count on days 3-5 to assess the risk of heparin-induced thrombocytopenia (HIT)
- LMWH treatment typically does not require laboratory monitoring of efficacy in routine practice [Bounameaux, 2004]
- the anticoagulant effect can be assessed by measuring anti-Xa activity, especially in the following cases: [Aguilera, 2016]
- very low- and very high-weight individuals (standard dose effects are unpredictable)
- BMI > 40 / weight > 150 kg, control at 48 and 96 hours
- women < 45kg and men < 57kg
- patients with renal insufficiency (as LMWH is excreted by the kidneys)
- CrCl < 30 mL/min (0.5 mL/s)
- pregnancy (weight gain with the risk of underdosing)
- patients at high risk of complications (thromboembolism/bleeding)
- very low- and very high-weight individuals (standard dose effects are unpredictable)
- correct results from anti-Xa testing depend on proper sample collection
- collect blood 3-4 hours after subcutaneous LMWH administration (at the time of plasma peak levels) after achieving steady-state (≥ 48h of LMWH application)
- collecting blood earlier or later results in lower values, potentially leading to overdosage
- ensure rapid, gentle injection with the careful aspiration of blood; prefilled citrate tubes should be filled to the mark
- avoid collecting blood from IV lines flushed with heparin if possible. If the blood must be drawn from an indwelling catheter, consider possible heparin contamination and sample dilution
- the line should be flushed with 5 mL of saline, and the first 5 mL of blood or six times the line volume should be drawn off and discarded before filling the coagulation tube
- for samples collected from a normal saline lock, twice the dead space volume of the catheter and extension set should be discarded
- the line should be flushed with 5 mL of saline, and the first 5 mL of blood or six times the line volume should be drawn off and discarded before filling the coagulation tube
- for larger larger blood samples, collect the biochemistry and CBC tubes first, followed by coagulation tubes
- mix the blood gently and transport it to the lab as quickly as possible; samples should be processed within 1 hour of collection
|
Reference range:
|
Contraindications
- hypersensitivity to the drug
- history of thrombocytopenia following LMWH administration
- active bleeding or increased risk associated with bleeding disorders (except DIC not caused by heparin)
- organic lesions with a tendency to bleed (e.g., peptic ulcer disease, recent eye or nervous system surgery)
- acute infective endocarditis
- severe renal impairment (creatinine clearance < 30mL/min)
Complications of LMWH treatment
-
bleeding complications → neutralizing effect of LMWH
-
injection site reactions
-
elevated liver enzymes
- allergic reaction
-
- less common with LMWH than with UFH
-
risk of osteoporosis with long-term use (> 6 months); the risk is lower than with heparin (UHF) , 2016)