ADD-ONS / MEDICATION / ANTICOAGULANTS
Rivaroxaban (Xarelto)
Updated on 22/04/2024, published on 24/10/2022
Rivaroxaban (XARELTO, KARDATUXAN) belongs to a group of drugs called direct oral anticoagulants (DOACs). It is a direct Factor Xa inhibitor and is used as an alternative to warfarin for the treatment and prevention of VTE and stroke prevention in people with nonvalvular atrial fibrillation (NVAF). Unlike warfarin, it does not require laboratory monitoring or special dietary restrictions.
Indications
- Primary and secondary stroke prevention in non-valvular atrial fibrillation (NVAF) – ROCKET AF a XANTUS trials
- Treatment and prevention of venous thromboembolism (VTE) – deep vein thrombosis (DVT) and pulmonary embolism (PE) – EINSTEIN trial
- A postoperative thromboprophylaxis in the knee and hip surgery
- Secondary prevention after acute coronary syndrome (ACS) or peripheral arterial disease (PAD) – as a low-dose add-on to clopidogrel and aspirin therapy – COMPASS trial
Off-label use:
-
treatment of acute heparin-induced thrombocytopenia (HIT) as initial therapy in hemodynamically stable patients or after initial therapy with a non-heparin parenteral anticoagulant
- patients with non-valvular atrial fibrillation (NVAF) who have undergone percutaneous coronary intervention with stent placement
-
acute symptomatic superficial vein thrombosis
Contraindications
- hypersensitivity to rivaroxaban (or any of the ingredients)
- pregnancy and lactation (DOACs are not recommended)
- not enough data are available to judge the safety and efficacy of DOACs used during pregnancy
- the drug is excreted into milk (probably only <10%) – not recommended
- severe renal impairment → use Cockcroft-Gault formula!
- all xabans are contraindicated with CrCl <15 mL/min (0.25 mL/s)
- active bleeding
- congenital or acquired lesions/conditions considered a significant risk factor for major bleeding:
- active or recent GI ulceration, presence of malignancy
- a recent brain or spinal cord injury or surgery
- eye surgery, vascular retinopathy
- recent intracranial hemorrhage
- known or suspected esophageal varices, AVMs, vascular aneurysms, or other vascular anomalies (relative contraindication)
- bronchiectasis or history of pulmonary bleeding
- uncontrolled severe arterial hypertension
- spontaneous/pharmacologic (other anticoagulants) bleeding disorders
- liver disease
- all DOACs are contraindicated in patients with liver disease associated with coagulopathy and clinically apparent risk of bleeding
- avoid rivaroxaban in patients with moderate-severe hepatic impairment (Child-Pugh classes B and C) or patients with coagulopathy associated with liver disease
- no dosage adjustment is necessary for patients with mild hepatic impairment
- concomitant therapy (especially potent P-glycoprotein inhibitors)
- ketoconazole, itraconazole
- macrolides
- conditions requiring VKAs
- avoid DOACs in patients with a mechanical valve or moderate-severe mitral stenosis (contraindication)
- not recommended in patients with prosthetic heart valves or rheumatic heart disease (however, rivaroxaban is non-inferior to warfarin in bioprosthetic MI valves according to the RIVERtrial)
- antiphospholipid syndrome (APS)
- avoid DOACs in patients with a mechanical valve or moderate-severe mitral stenosis (contraindication)
- caution is recommended in patients ≥ 75 years of age due to an increased risk of GI bleeding compared to warfarin and reported rates with other DOACs when used for long-term treatment
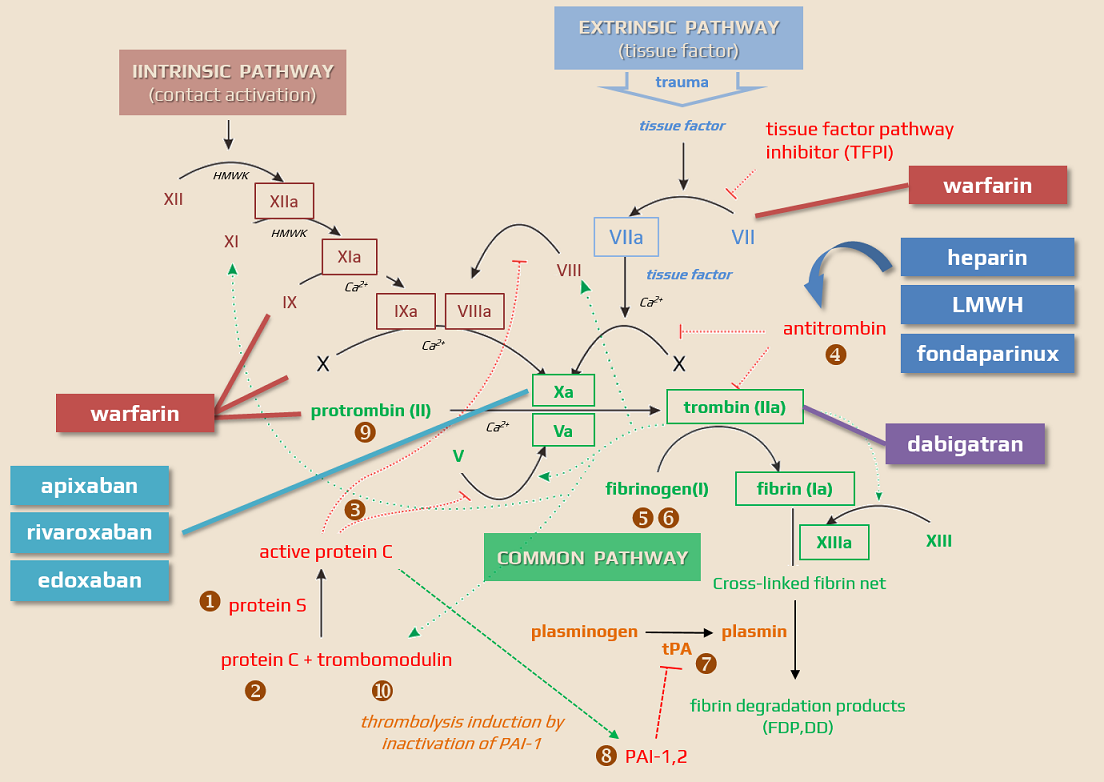
Mechanism of action
- direct, competitive, highly selective inhibitor of activated factor Xa (fXa)
- does not affect platelets
- unlike indirect factor Xa inhibitors (heparin, LMWH), rivaroxaban inhibits both free and clot-bound factor Xa; the effect is independent of the presence of AT III or other cofactors
- prevents the progression of the coagulation cascade through the final common pathway, thus preventing thrombin generation
| Direct anticoagulants They inactivate the clotting factors present in the plasma |
Indirect anticoagulants They affect clotting factors by reducing their liver production |
|
| Direct thrombin/factor Xa inhibitors These drugs bind to thrombin/factor Xa and thereby block their function |
Indirect thrombin/factor Xa inhibitors These drugs activate antithrombin |
|
|
||
|
||
|
||
Pharmacokinetics and pharmacodynamics
- active drug with an easy and rapid absorption
- maximum plasma concentration is reached within 2-4 hours after tablet intake
- half-life: 5-9 hours in young subjects and 11-13hours in older adults ((Mueck, 2014)
- must be taken with food
- high bioavailability (80-100%) and high albumin binding
- excretion is mainly via urine 66% and partially via feces 28%
- 1/3 of the dose is excreted unchanged by the kidneys
- and 2/3 is excreted in the kidneys and feces after metabolization in the liver (via CYP3A4, CYP2J2)
- in case of an overdose, activated charcoal can affect the absorption + selective antidote is available (andexanet); rivaroxaban is not dialyzable
- do not administer rivaroxaban to patients with CrCl < 0.25mL/s (15 mL/min)
- prolongs INR and PT; however, these tests can not be used to monitor the effect reliably
- rivaroxaban does not inhibit cytochrome P450 enzymes or known drug transporter systems and has no clinically relevant interactions with most commonly prescribed drugs
Interactions
- no significant interaction with atorvastatin, omeprazole, digoxin, or midazolam
- patients at risk:
- with renal insufficiency
- with hepatopathy-related coagulopathy ⇒ avoid DOACs!
| Content available only for logged-in subscribers (registration will be available soon) |
Administration and dosing
- rivaroxaban is administered orally once daily (every 24 hours) with food
- no dose adjustment is required in patients with CrCl >50 mL/min
- for patients with a BMI >40 or weight >120 kg; the International Society on Thrombosis and Hemostasis (ISTH) 2016 guideline suggests avoiding the use of rivaroxaban due to the lack of clinical data in this population. If used, ISTH recommends measuring peak and trough levels with an anti-factor Xa assay
| rivaroxaban (XARELTO) | stroke prevention | VTE prevention and therapy (no LMHW bridging required) |
VTE prevention in the hip or knee surgery (THR, TKR) |
| CrCl > 0.83 ml/s (>50 ml/min) | 20 mg once daily | 2×15 mg for 3 weeks then 20mg once daily (after 6 months, continue with 10 mg once daily if needed) |
10 mg once daily |
| CrCl 0.25-0.83 ml/s (15-49 ml/min) |
15 mg once daily |
2×15 mg for 3 weeks then 15 mg once daily |
Monitoring
- clinical monitoring usual for all anticoagulated patients
- specific laboratory monitoring is helpful in selected cases
Complications, adverse events
Bleeding
- bleeding (> 10%) is the most common AE: epistaxis, GI bleeding, hematuria or bleeding at the surgical site, puncture, or catheter site
- intracranial bleeding is less common compared to warfarin
- GI bleeding is more frequent compared to warfarin
- risk factors for bleeding:
- bacterial endocarditis
- underlying congenital or acquired bleeding disorders
- vascular retinopathy
- thrombocytopenia
- recent procedure/surgery, stroke, neuraxial procedures
- uncontrolled hypertension
- renal impairment
- recent major bleeding
- concomitant use of other drugs that affect hemostasis
- advanced age
- consider early (within 1-4h) administration of activated oral charcoal
- there is no hard evidence regarding the efficacy of antifibrinolytics (tranexamic acid, aminocaproic acid) or systemic hemostatic agents (desmopressin, aprotinin)
- the antidote is still somewhat a theoretical possibility (price, availability) → andexanet alfa
- andexanet alfa (trade name Andexxa) is an antidote for rivaroxaban and apixaban when reversal of anticoagulation is required
- andexanet alfa (trade name Andexxa) is an antidote for rivaroxaban and apixaban when reversal of anticoagulation is required
- antidote or substitution therapy should be reserved for severe bleeding or urgent surgery (↑ risk of VTE)
| ROCKET AF trial (median duration of treatment 19 months, total duration of treatment 41 months) |
||
| rivaroxaban | warfarin | |
| principal safety point (major + non-major clinically relevant bleeding) |
1475 (20.7%) |
1449 (20.3%) |
| major bleeding |
395 (3.6%) | 386 (3.45%) |
| critical bleeding |
91 (1.3%) |
133 (1.9) |
| fatal bleeding |
27 (0.4%) | 55 (0.8%) |
| intracranial bleeding | 55 (0.8%) | 84 (1.2%) |
| decrease in hemoglobin ≥ 2g/dl |
305 (4.3%) | 254 (3.6%) |
| non-major bleeding |
1185 (16.7%) | 1151 (16.2%) |
Other adverse events
- hematologic:
- anemia – often due to bleeding
- mild, usually transient thrombocytopenia
- GI disorders: abdominal pain
- dermatologic: pruritus, skin blisters
- CNS: dizziness, headache, insomnia, anxiety
- hepatic: serum transaminases elevated >3 x ULN, cholestasis
- neuromuscular & skeletal: back pain and muscle spasm
- bronchiectasis and pulmonary hemorrhage
- other adverse effects are mild and rare
Overdose
- if rivaroxaban was administered within the past 2 hours, consider activated oral charcoal (recommendation with unproven clinical benefit)
- antidote or substitution therapy should be reserved for severe bleeding or urgent surgery (due to ↑ risk of VTE)