ADD-ONS / ANTICOAGULANT THERAPY
Idarucizumab (PRAXBIND)
Updated on 02/06/2024, published on 10/11/2021
- the use of idarucizumab as an antidote to reverse the anticoagulant effects of dabigatran was approved by the FDA in October 2015 [Cassels, 2015]
- it has a 300-fold greater affinity for thrombin than does dabigatran
- indicated for adult patients treated with dabigatran (PRADAXA) when rapid reversal of anticoagulant effects is needed:
- urgent surgery or procedures (including intravenous thrombolysis)
- life-threatening or uncontrolled bleeding (including ICH)
- virtually no contraindications (except allergy), relevant drug interactions, or adverse events (AEs)
Mechanism of action
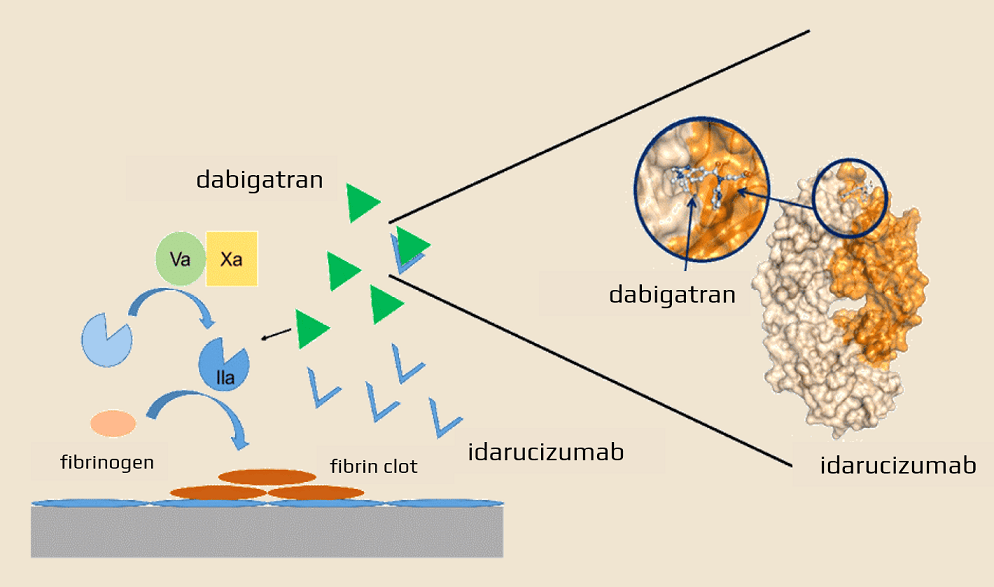
- idarucizumab is a fragment of a humanized monoclonal antibody that acts as a specific reversal agent for dabigatran
- it forms a tight complex with dabigatran and its acyl glucuronide metabolites, thereby immediately neutralizing the anticoagulant effect of dabigatran
- dabigatran has a 300-fold higher affinity for idarucizumab than for thrombin
- no interference with other anticoagulants and no secondary hypercoagulable state is induced
- administered as an IV bolus with rapid onset of action
- allows for the rapid resumption of anticoagulant therapy (within 24 hours post-administration)
- several pathways contribute to antibody metabolism; all involve the biodegradation of antibodies into smaller molecules, which are subsequently reabsorbed and incorporated into general protein synthesis
Clinial trials
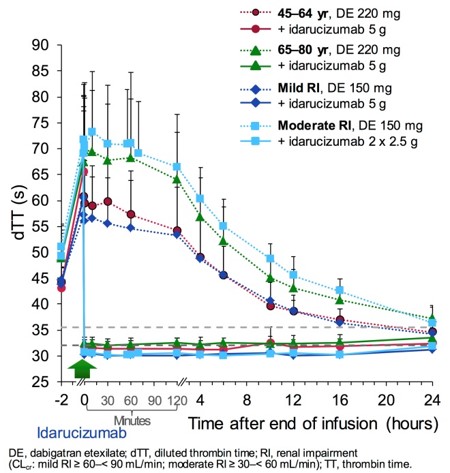
- RE-VERSE AD trial with idarucizumab at a dose of 5g IV
- effective within minutes
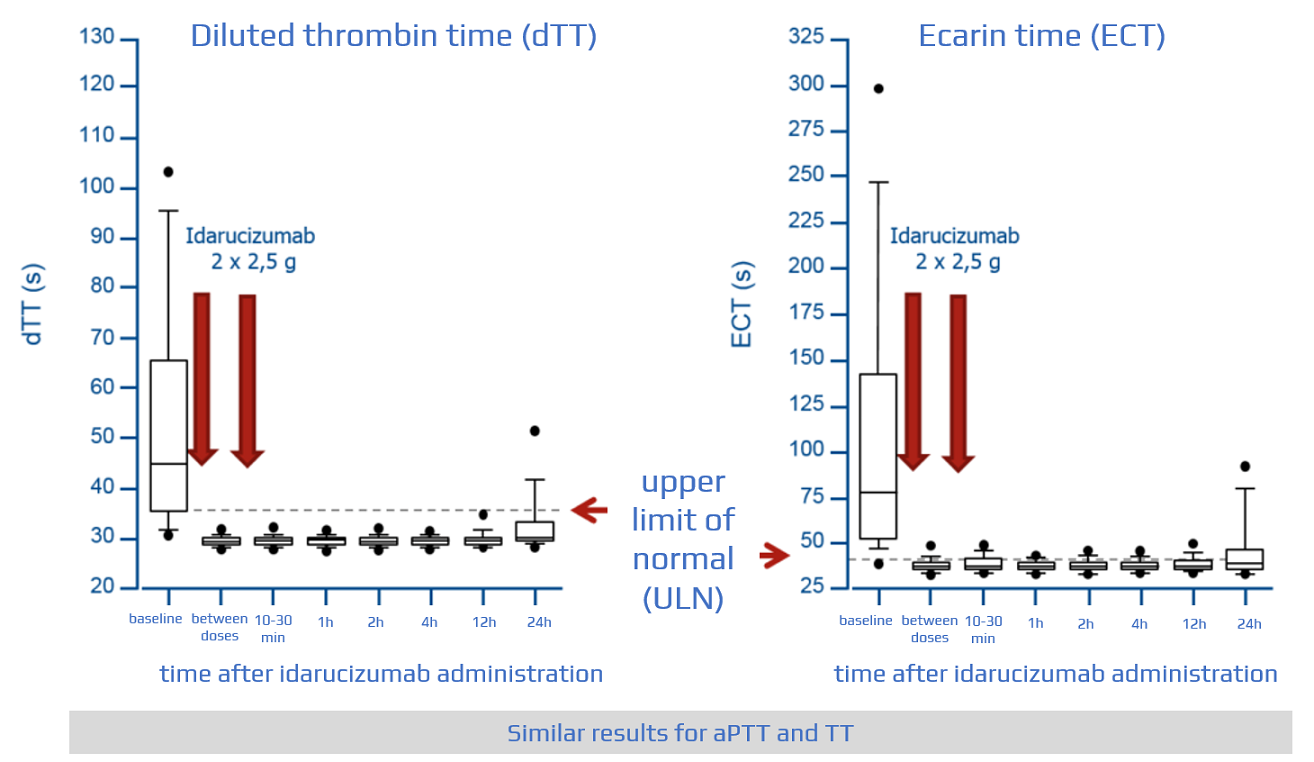
- analysis of 90 patients showed a reliable reduction of anticoagulant activity, achieving normal coagulation in 90% of patients at 4 and 12 hours → see here
- growing clinical experience exists with idarucizumab administration before thrombolysis and in the acute treatment of intracranial hemorrhage (ICH) → see here
Contraindications and precautions
- allergy to idarucizumab or its ingredients
- patients with hereditary fructose intolerance may be at risk of adverse reactions (such as hypoglycemia, hypophosphatemia, metabolic acidosis, elevated uric acid, and acute liver failure)
- no adequate and well-controlled studies in pregnant women
- it is not known whether idarucizumab can cause fetal harm when administered to a pregnant woman ⇒ should be given only if absolutely necessary
- no data available regarding its use during labor and delivery
- it is unknown whether idarucizumab passes into human milk
- no data are available on the effects of idarucizumab on nursing infants or on milk production
- the benefits of breastfeeding should be weighed against the mother’s clinical need for the drug (the benefits must outweigh the risks)
Idarucizumab administration
- aseptic handling is required during the preparation
- visually inspect for particulates and discoloration before infusion
- once the solution is withdrawn from the vial, administration should begin immediately; vials may be stored at room temperature (25°C /77°F) but must be used within 6 hours
- do not mix with other medications
(Praxbind)
-
- vial = 2.5g/50 mL
- administer a 5g dose intravenously as two consecutive 2.5 g infusions or bolus injections
- an existing intravenous line may be used; flush with sterile 0.9% NS solution before infusion
- no other infusion should be administered simultaneously via the same IV access
- renal impairment does not affect the reversal effect of idarucizumab ⇒ no dose adjustment is required
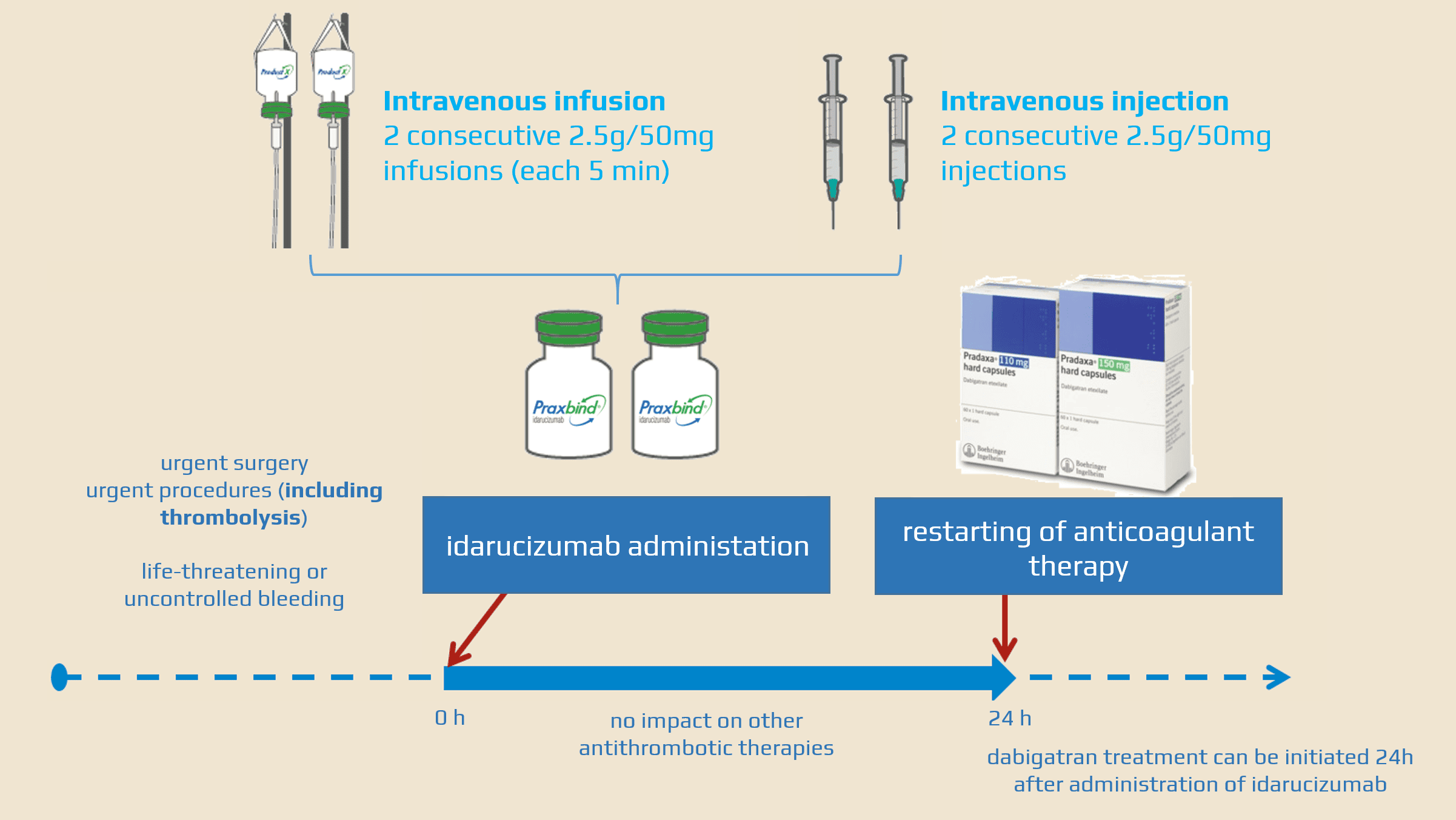
Resuming antithrombotic therapy
- patients treated with dabigatran have underlying conditions predisposing them to thromboembolic events
- consider resumption of anticoagulant therapy ASAP to reduce this risk
- idarucizumab does not interfere with other anticoagulant or antithrombotic therapies
- dabigatran treatment can be resumed 24 hours after idarucizumab administration
Praxbind and intravenous thrombolysis
- intravenous thrombolysis (IVT) in acute stroke patients on dabigatran is possible with dabigatran therapy if aPTT + TT, ECT, or HEMOCLOT values do not exceed upper limits of normal (AHA/ASA 2013 III/C)
- a normal aPTT alone is not sufficient as it does not completely rule out an elevated TT [Kermer, 2017] [Hankey, 2014]
- IVT may be considered with normal TT or TT <60 s AND normal APTT (ESO guidelines 2021)
- expert opinion only; insufficient evidence to make an evidence-based recommendation
- Hemoclot – a dabigatran level < 50 ng/ml > 6h after the last dose of the drug indicates the absence of anticoagulant activity
- administration of thrombolysis after prior neutralization with PRAXBIND is possible (IVT is an emergency procedure); registry data suggest that administration of the antidote PRAXBIND in patients with acute stroke followed by thrombolysis is effective, safe, and easy to perform [Kermer, 2017]
- expert consensus suggests a combination of idarucizumab and IVT for patients with acute ischemic stroke of <4.5 hours duration (ESO guidelines 2021) [Diener, 2017]
Adverse events
- common side effects
- hypokalemia (7%)
- delirium (7%)
- constipation (7%)
- fever (6%)
- pneumonia (6%)
- headache (5%)
- serious side effects:
- thrombosis/thromboembolism – patients are at increased thrombotic risk due to the underlying condition necessitating dabigatran prescription
- recurrent bleeding (some patients may require an additional dose of idarucizumab)
- hypersensitivity reactions (severe allergic reaction – fever, respiratory distress due to airway spasms, hyperventilation, rash, and pruritus)
- thrombosis/thromboembolism – patients are at increased thrombotic risk due to the underlying condition necessitating dabigatran prescription
- patients with hereditary fructose intolerance may be at risk for adverse effects such as hypoglycemia, hypophosphatemia, metabolic acidosis, hyperuricemia, and acute liver failure