ISCHEMIC STROKE / ACUTE THERAPY
Intravenous thrombolysis in acute stroke
Updated on 25/07/2024, published on 25/10/2023
All patients with suspected acute stroke or transient ischemic attack (TIA) require immediate clinical evaluation to establish the diagnosis and to determine eligibility for intravenous thrombolysis (IVT) and/or endovascular thrombectomy (EVT)
- intravenous thrombolysis (IVT) is the standard therapy for acute ischemic stroke → fibrinolytic drugs
- a clear benefit of treatment was repeatedly demonstrated; NNT to achieve mRS 0-1 varies in different publications
- 0-3h window – NNT 10, 3-4.5h time window – NNT 19 [Brunström, 2015]
- 0-3h window – NNT 8, 3-4.5h time window – NNT 12 [Tsivgoulis, 2021]
- a clear benefit of treatment was repeatedly demonstrated; NNT to achieve mRS 0-1 varies in different publications
- don´t skip IVT in eligible patients if a thrombectomy is planned
- both alteplase and tenecteplase can be used
- both drugs are tissue plasminogen activators (tPA)
- historically, the term “tPA” has been used to refer to alteplase because it has long been the only tissue plasminogen activator in use; nowadays, the name of the specific agent should be used
- start IVT as soon as possible; the earlier the thrombolysis is started, the greater the chance of achieving recanalization and the lower the risk of ICH, mortality, and dependency [Emberson, 2014]
- planned endovascular treatment is not a reason to avoid IVT
- non-randomized trials suggest better outcomes with combination therapy [Mistry, 2017] [Goyal, 2018]
- the results of SWIFT-DIRECT and DIRECT-SAFE trials do not support omitting IVT before thrombectomy in eligible patients
- mechanical thrombectomy should not prevent the initiation of IVT, and IVT should not delay mechanical thrombectomy (do not wait for potential clinical/radiological/neurosonological improvement)
- IVT can be performed concomitantly with EVT, and there is insufficient evidence for premature termination of IVT before the initiation of EVT
- transport from the Stroke Center to the Comprehensive Stroke Center should happen immediately after the initiation of IVT
- the benefit of IVT before MT in patients with ASPECTS < 6 is uncertain (Broocks, 2022)
- the Wake-Up Stroke (WUS) within 4.5 hours after symptom recognition after wakeup ⇒ IVT + MT (ESO guidelines 2022)
- the selection criteria for IVT and MT in patients with WUS (ESO guidelines 2021)
- core < 70 ml (rCBF <30% on CTP or ADC < 620 μm2/s)
- mismatch ratio (penumbra/core) > 1.2 – penumbra defined as Tmax > 6s
- mismatch volume > 10 ml
- the thrombolysis-eligible patient should preferably be treated at the Stroke Center or Comprehensive Stroke Center (CSC) and should be admitted to the ICU for at least the first 24 hours after IVT initiation
- in the stroke center, the availability of emergency transport to the CSC (to perform endovascular treatment) should be arranged before starting IVT in patients with LVO
Thrombolytics
- alteplase and tenecteplase are the only fibrinolytic drugs recommended for intravenous thrombolysis (IVT) in acute stroke patients (ESO guidelines 2021)
- ESO guidelines 2023 suggest favoring tenecteplase 0.25 mg/kg over alteplase 0.9 mg/kg in <4.5 h time window (because of safety and efficacy data and because tenecteplase can be administered with a single bolus)
Alteplase (ACTILYSE)
- a tissue plasminogen activator (tPA) that has long been the standard treatment for acute ischemic stroke
- in a 0-4.5 hour window prefer the standard dose (0.9 mg/kg) to a reduced dose (ESO guidelines 2021)
- 10% is given as an IV bolus over 2 minutes, followed by a 1-hour infusion
Tenecteplase (METALYSE)
- a genetically modified tPA with enhanced fibrin specificity and longer half-life, allowing bolus administration
- a safe and effective alternative to alteplase in a 4.5h time window (ESO guidelines, 2023)
- tenecteplase 0.25 mg/kg administered as a single bolus is preferred over alteplase (ESO guidelines 2023)
- patients scheduled for thrombectomy
- the EXTEND-IA TNK trial showed a higher incidence of reperfusion and better functional outcome with tenecteplase compared to alteplase in patients with large artery occlusion (LVO) followed by MT (complete reperfusion 22% with TNK vs. 10% with alteplase)
- a meta-analysis of 4 RCTs showed greater efficacy of tenecteplase versus alteplase in patients with LVO [Katsanos, 2020]
- patients scheduled for thrombectomy
-
- standard acute stroke patients eligible for IVT
- randomized trials ACT (2022) and ATTEST-2 (2023) showed non-inferiority of TNK at a dose of 0.25 mg/kg compared to alteplase in a standard indication
- a meta-analysis of 5 trials demonstrated the non-inferiority of TNK versus alteplase [Burgos, 2019]
- according to registry data, TNK 0.25 mg/kg is associated with a lower risk of sICH compared to alteplase → see here
- standard acute stroke patients eligible for IVT
-
- wake-up stroke
- tenecteplase at a dose of 0.25 mg/kg may be used instead of alteplase provided specific imaging criteria are met (ESO guidelines 2023, expert opinion)
- tenecteplase at a dose of 0.25 mg/kg may be used instead of alteplase provided specific imaging criteria are met (ESO guidelines 2023, expert opinion)
- wake-up stroke
- tenecteplase at a dose of 0.40 mg/kg is not recommended (ESO 2023)
- NOR-TEST 2 trial part A with TNK dose of 0.4 mg/kg was stopped prematurely for safety reasons (TNK did worse than alteplase)
- in ExTEND-IA trial part 2, a dose of 0.40 mg/kg versus 0.25 mg/kg did not significantly improve reperfusion before MT (Cambell, 2020)
ALTEPLASE
- NINDS – the effect was demonstrated within 3h after stroke onset – mRS 0-1/3months.: 39% vs. 26% (placebo)
- ECASS 3 – effect demonstrated within 4.5h window after stroke onset
- SITS-MOST – effect within a 4.5 hours window
- analysis of SITS online registry data
- comparable results from routine clinical practice with results of randomized trials
- EXTEND (2018)
- time window 4.5-9h, CTP mismatch ratio >1.2 + mismatch ≥ 10 ml
- mRS 0-1 /3m RR 1.44 (35.4% vs 29.5%)
- the efficacy and safety of IVT indicated by CTP were also demonstrated in a meta-analysis of the EXTEND, ECASS-4/EXTEND, and EPITHET (mRS 0-1, OR 1.86) [Campbell, 2020].
- WAKE-UP
- MR DWI/FLAIR mismatch
- OR 1.61 (mRS 0-1 53.3 vs 41.8)
- the effect is also present with lacunar infarcts
- MR DWI/FLAIR mismatch
- MR WITNESS
TENECTEPLASE
- NOR-TEST (bolus 0.4 mg/kg, maximum 40mg vs. alteplase 0.9 mg/kg)
- similar results as alteplase
- similar results as alteplase
- ACT (2022) and ATTEST-2 (2023) showed non-inferiority of TNK at a dose of 0.25 mg/kg compared to alteplase in a standard indication
- EXTEND-IA TNK
- TNK 0.25 mg/kg versus alteplase within 4.5 h
- large artery occlusion (LVO); TNK administered before subsequent mechanical thrombectomy
- better results than alteplase
- meta-analysis of 4 RCTs in patients with LVO [Katsanos, 2020]
- TNK vs. alteplase – mRS 0-2 OR 2.06, recanalization 3.05
- NOR-TEST 2 part A (Norwegian Tenecteplase Stroke Trial 2)
- prematurely terminated
- TNK 0.4 mg/kg vs. alteplase in moderate/severe ischemic stroke
- tenecteplase resulted in worse safety- and functional outcomes than alteplase did
Advantages and disadvantages of IVT
- no special equipment is required
- quick initiation of therapy
- low costs
- short therapeutic window
- low efficacy in large thrombosis (→ Clot Burden Score)
- larger artery occlusion = lower efficacy
- complete recanalization in terminal ICA occlusion ~10%, M1 ~20-30%
- recanalization rate (TIBI 2b/3) in Medium-sized Vessel Occlusion (MeVO – mostly M2 segment) is approx. 42% [Ospel, 2020]
- according to the INTERRSECT study, recanalization of intracranial ICA and MCA occlusions is achieved in 27% of patients [Ohara, 2020]
- bleeding risk (intracranial, but also systemic bleeding)
- various definitions of symptomatic IC bleeding; ~ 6-7% according to the NINDS definition → classification of hemorrhagic complications
- risk is lower in the posterior circulation [Keselman, 2020]
- various definitions of symptomatic IC bleeding; ~ 6-7% according to the NINDS definition → classification of hemorrhagic complications
- numerous contraindications (many have become relative over time) [Tsivgoulis, 2021]
Indications for intravenous thrombolysis
Time from stroke onset (AHA/ASA and ESO guidelines)
< 4.5 h
- age ≥ 16 years
- the risk/benefit of treatment in individuals < 16 years of age is unknown
- since 2019, Actilyse has been approved in patients aged 16-17 years based on SITS-ISTR registry data
- the upper age limit of 80 years has been removed (ESO guidelines 2021)
- according to metanalysis, IVT is safe in patients > 80 years of age [Bluhmki, 2020]
- age alone should not be a limiting factor for IVT (even in wake-up stroke, an ischemic stroke of 4.5–9 h duration with CT or MRI mismatch, minor stroke with disabling symptoms, etc.)
- both ACTILYSE 0.9 mg/kg for patients without subsequent EVT and METALYSE 0.25 mg/kg can be used
-
tenecteplase is favored over alteplase (ESO guidelines, 2023)
- alteplase dose reduction is not recommended
-
- initial non-contrast brain CT is normal or shows early ischemic changes of small or moderate extent (<1/3 MCA territory or ASPECTS ≥7)
- even in the presence of extensive early ischemic changes, IVT may be performed after careful risk-benefit consideration, but a worse outcome and higher risk of bleeding should be expected (ESO Guidelines 2021)
- IVT should not be performed in the presence of extensive marked hypodensities
> 4.5 h non-selected
- IVT is accepted for basilar artery occlusion (BAO) within 24 after stroke onset without the use of advanced imaging (ESO/ESMINT guidelines, 2024)
- for adults with ischemic changes being more extensive than those included in RCTs (ASPECTS 0-6), consider other prognostic variables (such as pre-stroke handicap, age, and frailty) before offering reperfusion therapy
- reperfusion therapy should be withheld in those with very extensive ischemic changes
4.5-9 h
- advanced brain imaging should be used to select patients for IVT (data for alteplase only) if they present between 4.5 and 9 h after the symptom onset or if a stroke is detected upon awaking from sleep (ESO guidelines 2021)
- CT/MRI perfusion mismatch – EXTEND (2018) criteria (ESO guidelines 2021)
- ≥ 18 years, premorbid mRS < 2, NIHSS 4-26
- time:
- 4.5-9 h after onset (if the time of onset is known)
- stroke present on awakening – IVT initiated within 9h of mid-sleep interval
- radiological parameters:
- mismatch >1.2 , absolute >10 ml (Tmax >6 s on perfusion)
- core < 70 ml – rCBF < 30% (CTP) or ADC < 620 mm2/s (DWI)
- results: mRS 0-1 /3m RR 1.44 (35.4% vs 29.5%)
- efficacy and safety were also demonstrated in a meta-analysis of the EXTEND, ECASS-4/EXTEND, and EPITHET trials (mRS 0-1, OR 1.86) [Campbell, 2020]
- MR DWI/FLAIR mismatch (according to WAKE-UP trial criteria)
- for patients without CT or MRI core/perfusion mismatch, the ESO 2021 guidelines suggest against IVT with alteplase
- if the patient is a candidate for both IVT and MT in the 4.5-9 h window (according to the above criteria), then:
- initiate IVT before transport from the stroke center to the Comprehensive Stroke Center (CSC) (ESO guidelines 2021)
- if the patient is in a CSC, there is no consensus on whether to start IVT before MT (ESO guidelines 2021)
-
tenecteplase 0.25 mg/kg could be a reasonable alternative to alteplase 0.9 mg/kg for patients eligible for IV thrombolysis after selection with advanced imaging (ESO 2023, expert consensus)
Wake Up Stroke (WUS) / Stroke of Unknown Time of Onset
- according to a meta-analysis of the WAKE-UP, EXTEND, THAWS, and ECASS-4 studies, IVT is safe and effective in patients with persisting salvageable brain tissue (determined by advanced CT/MR techniques) [Thomalla, 2020]
- therapy with tPA resulted in a higher percentage of patients with no or minor neurological deficit than the use of a placebo
- MR DWI/FLAIR mismatch – DWI lesion without a correlate on FLAIR, with a DWI lesion < 1/3 of the MCA territory (AHA/ASA 2019 IIa/B-R) (ESO guidelines 2021)
- in the absence of changes on FLAIR, the predicted stroke duration is < 4.5h – see positive results of the WAKE-UP trial
- CT/MR perfusion (perfusion mismatch on CT or MR – see EXTEND study criteria above) (ESO guidelines 2021)
- IVT administered within 9 hours of mid-sleep interval
- if the patient is a candidate for both IVT and EVT, then IVT is likely to be administered before EVT (ESO Guidelines 2021, expert consensus)
- indication for IVT based on ASPECTS score on NCCT – ESO guidelines 2021 recommend advanced imaging in the time window of > 4.5h or WUS
- however, it appears safe to thrombolyse patients with wake-up stroke and a normal NCCT; similar results have been reported in patients with witnessed-onset and wake-up strokes and normal CT (Armon, 2019)
- patients with WUS < 4.5h after awakening or patients with an unclear stroke onset time
- NCCT: normal findings or early ischemic changes in < 1/3 of the MCA territory and/or ASPECTS > 7
- non-randomized TRUST-CT trial showed benefit in patients with ASPECTS ≥ 7 (OR 1.94 ) – RCTs are needed
- however, it appears safe to thrombolyse patients with wake-up stroke and a normal NCCT; similar results have been reported in patients with witnessed-onset and wake-up strokes and normal CT (Armon, 2019)
+
A neurological deficit
Mild deficit → see comments on IVT contraindications
- mild disabling deficit (mild stroke – NIHSS 0-5, e.g., homonymous hemianopsia, mild aphasia or dysarthria in an actor, mild paresis in a pianist, etc.) ⇒ IVT is recommended (ESO guidelines 2021) (AHA/ASA 2019 I/B-R)
- mild non-disabling deficit: IVT is not recommended (ESO guidelines 2021) (AHA/ASA 2019 III/B-R)
- mild non-disabling deficit + LVO ⇒ IVT is suggested, but an individualized approach is required (ESO 2021 – expert consensus)
- neurological deficits may fluctuate; many patients with acute arterial occlusion will eventually progress if recanalization is not achieved (END – Early Neurological Deterioration)
- however, the TEMPO-2 trial showed no benefit and possible harm from treatment with IV tenecteplase ⇒ patients with minor stroke and intracranial occlusion should not be routinely treated with IVT
- rapidly improving but still disabling deficit ⇒ IVT is suggested (ESO 2021 – expert consensus)
- thrombolysis for central retinal artery occlusion (CRAO) → see separate chapter here
A severe stroke
- the benefit of IVT in patients with NIHSS > 25 is unclear; IVT may be considered a life-saving procedure (ESO guidelines 2021)
- the extent of early ischemic changes that could be considered futile for thrombolytic therapy is not clear
- however, ASPECTS < 7 has been shown to increase the risk of sICH [Singer, 2009]
- according to ESO guidelines 2021 (expert consensus), patients with NIHSS > 25 and/or ASPECTS < 7 should probably be treated with IVT
- selection criteria may include results of advanced imaging (especially core/perfusion mismatch), the extent of white matter lesions, other contraindications to IVT, and pre-stroke disability
- direct mechanical thrombectomy (dMT) is recommended
Administration of thrombolytic therapy
| Start thrombolysis immediately after the patient´s arrival at the hospital. No investigation should delay its initiation. The Door-To-Needle Time (DTN) should be less than 45-60 minutes; in uncomplicated patients, the bolus can usually be administered within 10-15 minutes. Urgently consult with a stroke specialist within the institution or through telestroke services if needed. |
Initial patient assessment and monitoring
- focus on ultra-fast administration of the IVT bolus (within 10-15 minutes in an uncomplicated patient!)
- the procedure requires logistical arrangements within the stroke center
- many centers no longer insert a urinary catheter, second IV cannula, or perform an ECG before IVT administration
- it is a good idea to check contraindications by phone or in the documentation before the patient arrives
- pre-hospital mobile stroke units are also being tested to expedite treatment. The cost-benefit is still uncertain; one analysis showed no better mRS 0 and 1 compared to standard care; however, there was no higher incidence of hematoma [Kunz, 2016]
- perform a rapid clinical examination including assessment of NIHSS and vital signs, verify the time of onset and contraindications → initial examination of an acute stroke patient
- perform non-contrast CT (NCCT) of the brain (sufficient for IVT indication)
- there is no known threshold for the extent of early CT signs of ischemia that should lead to withholding IVT unless there is a known contraindication
- hyperdense media sign is not a contraindication to IVT (AHA/ASA 2019 IIa/B-NR)
- routine use of MRI to exclude microbleeds is not recommended (AHA/ASA 2019 I/B-NR)
- always consider the risk-benefit of IVT (AHA/ASA 2019 I/C-EO)
- minimize the risk of stroke mimics (especially exclude hypoglycemia); imaging is helpful (especially CTA/CTP)
- do not wait for coagulation test results unless:
- there is reasonable suspicion of thrombocytopenia or a bleeding disorder (from patient history)
- anticoagulant use is uncertain but possible
- the patient is taking anticoagulants ⇒ follow a specific protocol for IVT in anticoagulated patients
- there is reasonable suspicion of thrombocytopenia or a bleeding disorder (from patient history)
- if no contraindication is found, administer alteplase/tenecteplase bolus
- proceed with CTA/CTP and consider thrombectomy
Infusion preparation, dose
Care during thrombolysis
- monitor vital signs
- blood pressure (BP) should be lowered and maintained below 180/105 mmHg before, during, and 24 hours after IVT administration (BP > 185/110 mmHg is a contraindication to thrombolytic treatment) (AHA/ASA 2022)
- check BP every 15 minutes in the first 2 hours, then every 30 minutes for the next 22 hours
- in cases of decompensated hypertension or parenteral antihypertensive therapy, measure more frequently (every 3-5 minutes) → protocol for BP correction during IV
- aggressive BP correction in the first 72 h (BPs < 130-140 mmHg) leads to a reduction in ICH risk (14.8% vs. 18.7%) according to the ENCHANTED study, yet therapy did not affect the outcome (mRS/3m). Patients with mild and moderate deficits were included in the study [Anderson, 2019])
- avoid abrupt BP drops because they might worsen or induce ischemia, particularly in the setting of intracranial or extracranial arterial occlusion/stenosis
- check BP every 15 minutes in the first 2 hours, then every 30 minutes for the next 22 hours
- monitor for bleeding complications (injection sites, oral cavity, GIT, genitourinary tract, site of injury) and allergic reactions, including angioedema (→ more here)
- monitor NIHSS / GCS
- if NIHSS increases by ≥ 4 points (which can not be attributed to, e.g., TCCD-demonstrated reocclusion) and in case of new-onset severe headache, consider stopping IVT (if alteplase is used) and order a CT scan
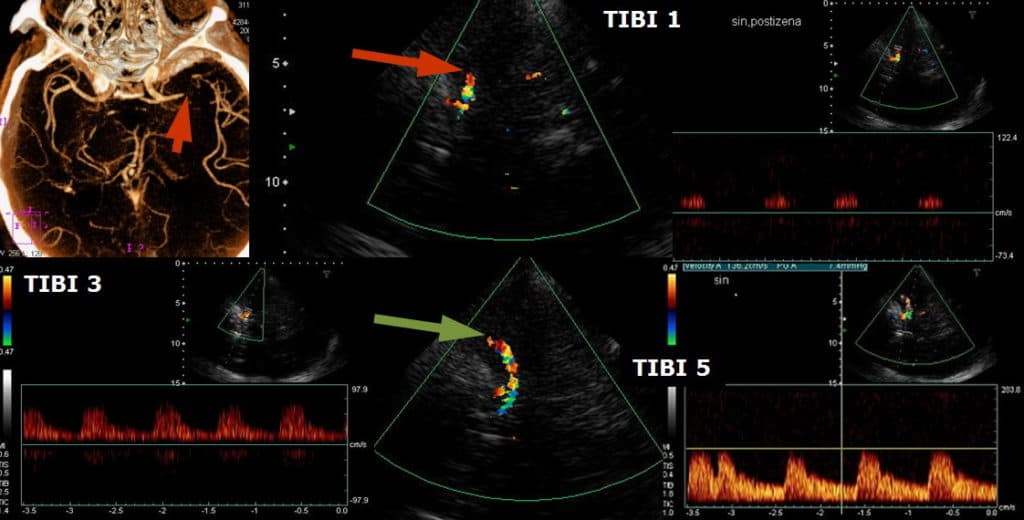
- TCD/TCCD monitoring
- repeat quick examinations with TIBI assessment only; continuous monitoring (sonothrombolysis) is not recommended beyond clinical trials
- in patients with large-artery occlusion (LVO), arrange endovascular intervention at the start of IVT and do not wait for the completion of IVT (the chance of LVO recanalization with IVT alone is low; <10% for terminal ICA occlusion)
- transport the patient to the center performing EVT immediately after the initiation of IVT
- transport the patient to the center performing EVT immediately after the initiation of IVT
- criteria of probable persistent occlusion:
- no clinical improvement (<40% NIHSS reduction)
- TCD/TCCD TIBI ≤ 3
Post-thrombolysis monitoring
- check blood samples
- CBC + coagulation (aPTT, INR, fibrinogen) at 6 and 24 h post-IVT
- basic metabolic panel at 12-24 h
- follow-up brain CT at 22-36 h
- continue strict BP control for the first 24h (< 180/105 mm Hg)
- insert urinary catheter either before thrombolysis or > 60 min after completion of IVT
- do not insert a nasogastric tube or central venous catheter (CVC), and do not perform arterial puncture in the first 24 h
- in emergencies, wait at least 30 minutes after infusion; APTT and INR must be normal
- prefer puncture of the femoral vein over the subclavian or jugular vein
- avoid IM injections during thrombolysis and 60 minutes after completion of IVT
- avoid anticoagulation therapy in the first 24 hours after IVT
- even prophylactic doses of LMWH should be delayed for 24h ⇒ use mechanical DVT prevention (IPC) instead
- antiplatelet therapy in the first 24 hours:
- do not administer IV aspirin within 90 minutes after starting IVT (ARTIS study) (AHA/ASA 2019 III/B-R)
- do not administer abciximab concomitantly with IVT
- standard antiplatelet therapy should be started after 24h if a hemorrhagic complication has been excluded by follow-up CT
- antiplatelet therapy may be administered within 24h after IVT if evidence of benefit in comorbidities exists (e.g., in a patient after acute stenting, with concomitant acute MI, etc.) (AHA/ASA 2019 IIb/B-NR)