ADD-ONS / SCALES
Max-ICH score
David Goldemund M.D.
Updated on 29/08/2023, published on 08/06/2022
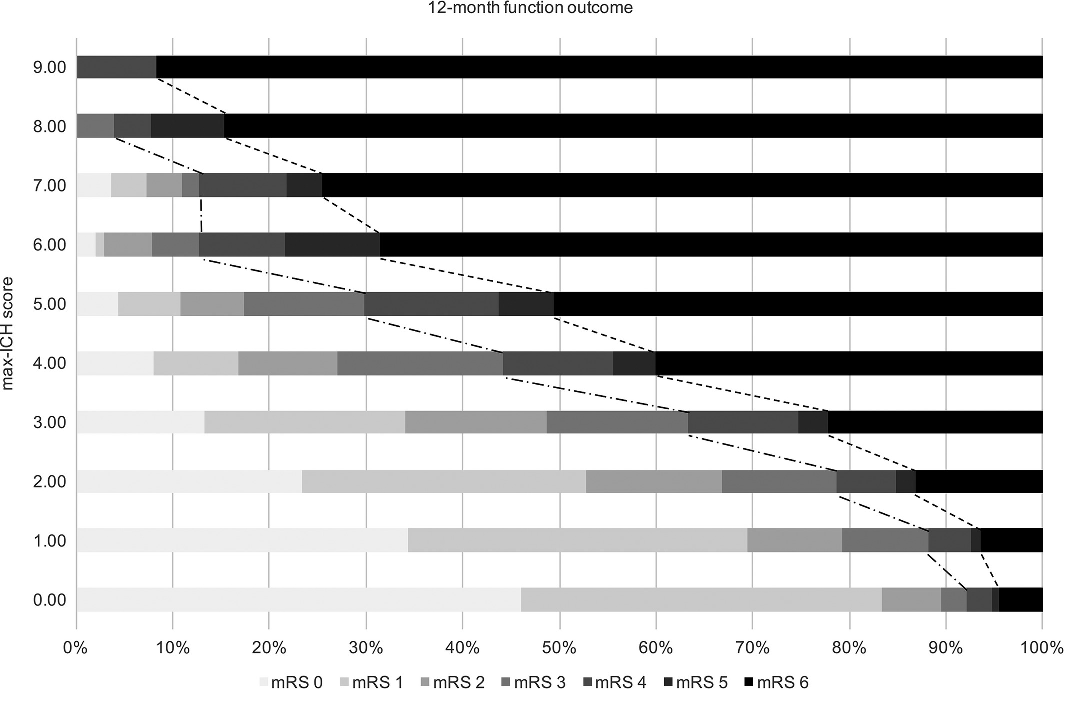
- simple, reliable tool for predicting unfavorable long-term (12-month) functional outcome and mortality after intracerebral hemorrhage (ICH)
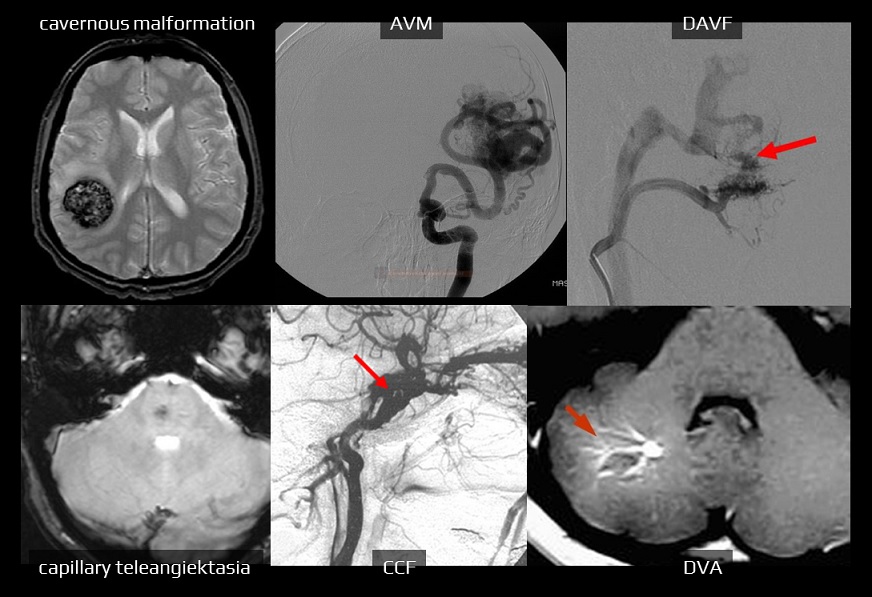
- ICH involving both lobar and non-lobar regions should be scored based on the location where the ICH most likely originated
- if 2 large ICHs occur simultaneously, more than 1 point referring to the ICH volume should be assigned
- the maximum total score for a single hematoma (with/without IVH) is 9 points
- each 1-point increase in the max-ICH score was associated with an OR of 1.24 for an unfavorable outcome (Suo, 2018)
- external validation comparing the ICH score and the max-ICH score shows a similar prognostic value (Schmidt, 2018)
|
Max-ICH score (0-9 points for a single ICH)
|
|
|
0-6
7-13
14-20
≥21
|
0
1
2
3
|
|
Age (years)
≤ 69
70-74
75-79
≥ 80
|
0
1
2
3
|
|
Lobar hematoma volume ≥ 30 mL
|
1
|
|
Non-lobar hematoma volume ≥ 10 mL
|
1
|
|
Intraventricular hemorrhage (IVH)
|
1
|
|
Oral anticoagulants
|
1
|
|
Calculation of hematoma volume
|
|
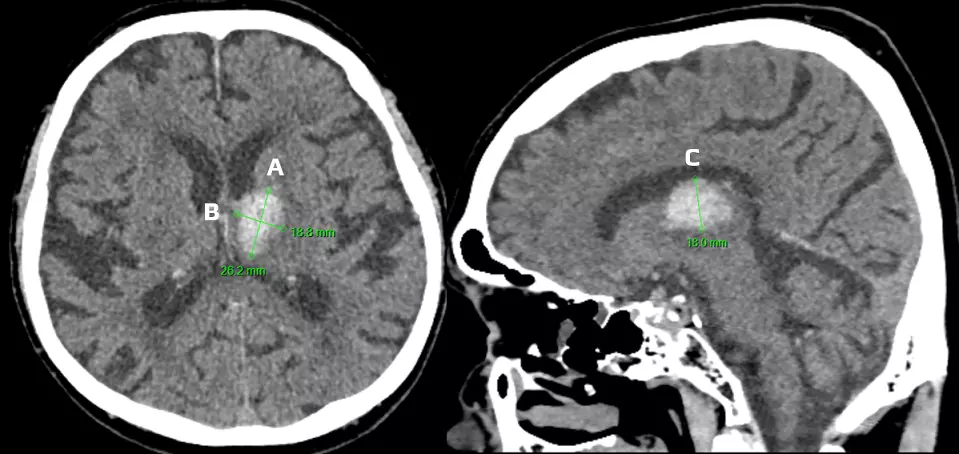
volume in mL (cm3) ≈ A x B x C /2 (round or ellipsoid shape)
volume in mL (cm3) ≈ A x B x C /3 (irregular, separated, or multinodular shape) [Huttner, 2006] |
|
A, B – hemorrhage width and length – see image (in cm)
C = height
|
|
For more precise measurement, count slices as follows:
|