ADD-ONS / SCALES
Clot Burden Score (CBS)
David Goldemund M.D.
Updated on 21/04/2024, published on 20/12/2022
- the extent of intracranial thrombosis predicts clinical outcome, final infarct size, and risk of hemorrhagic transformation in acute ischemic stroke
- clots with proximal localization and increased length are more difficult to treat and have a worse outcome
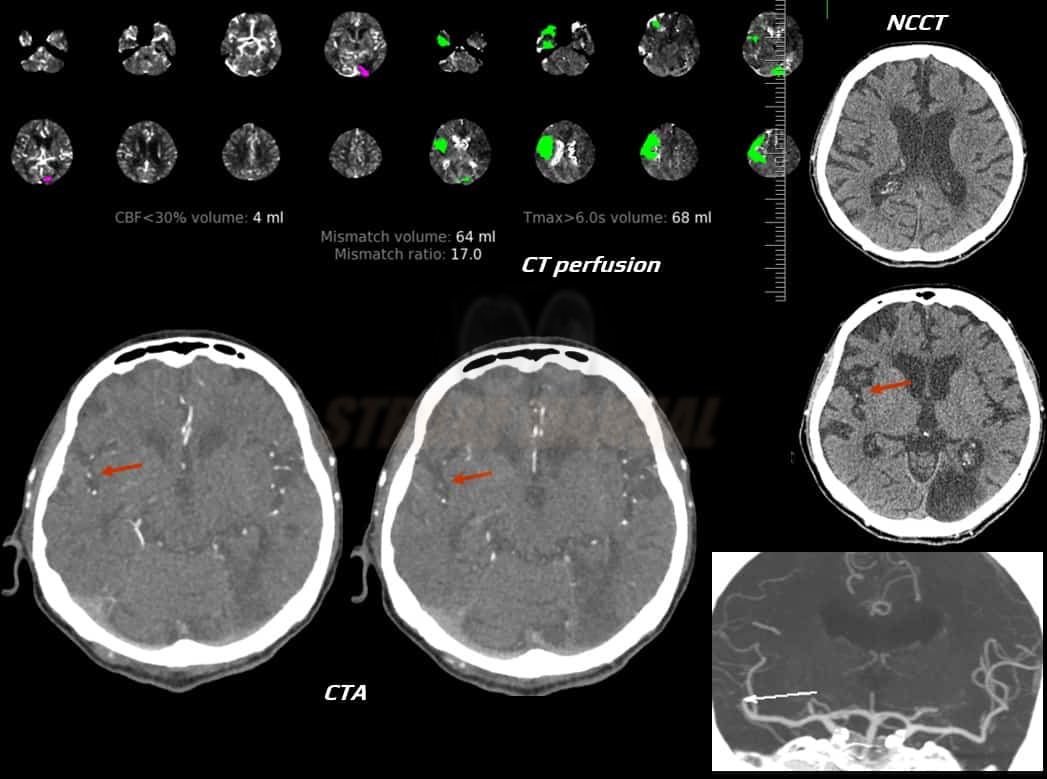
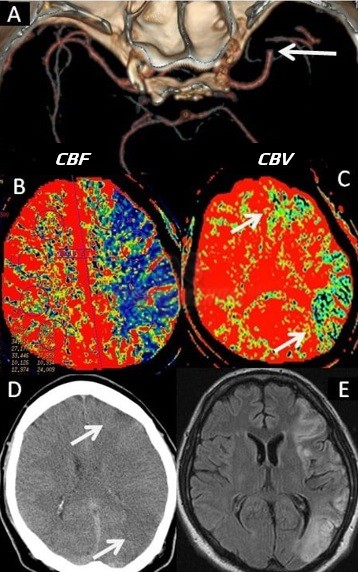
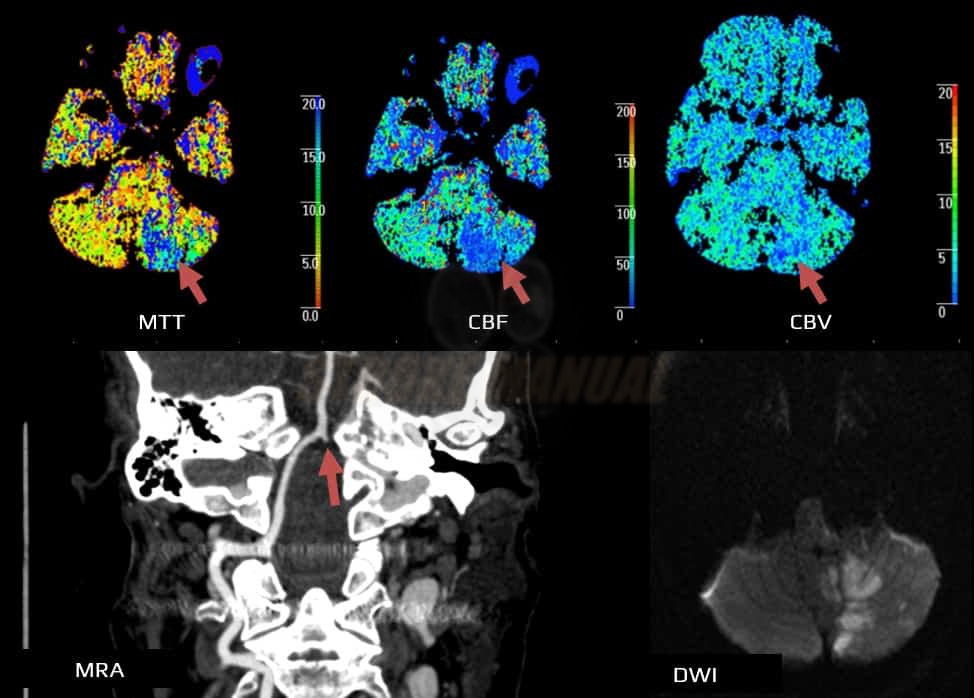
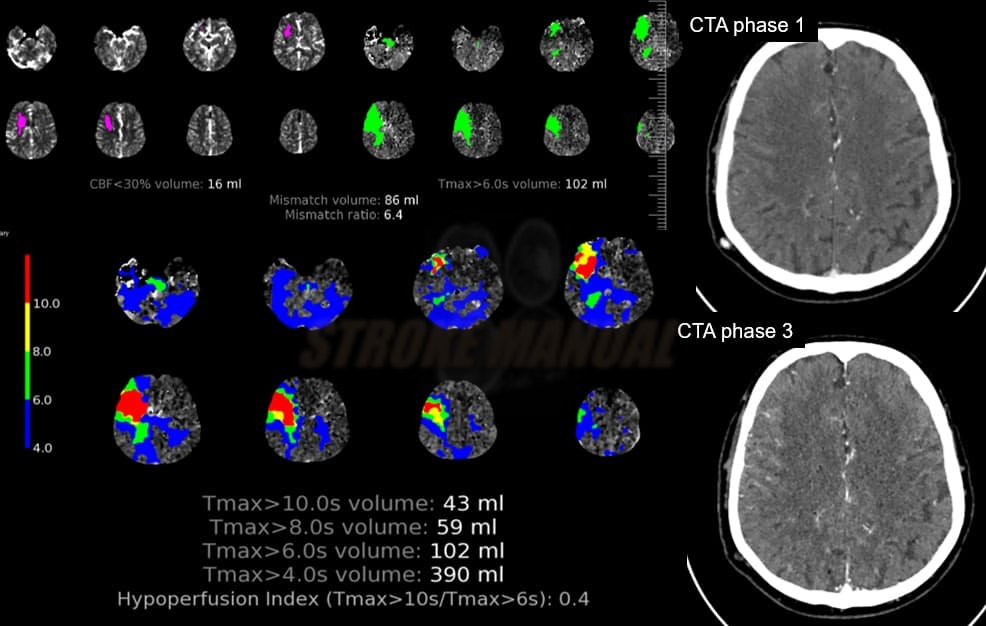
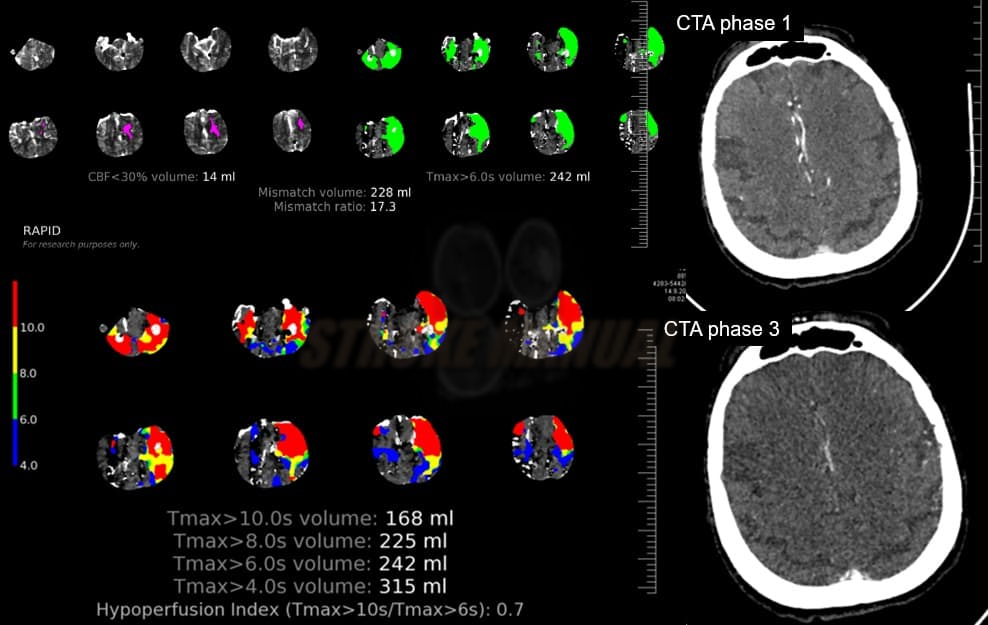
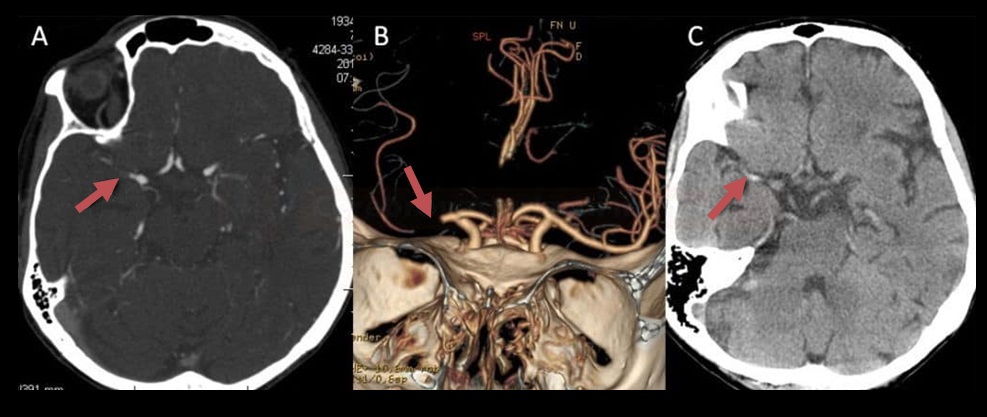
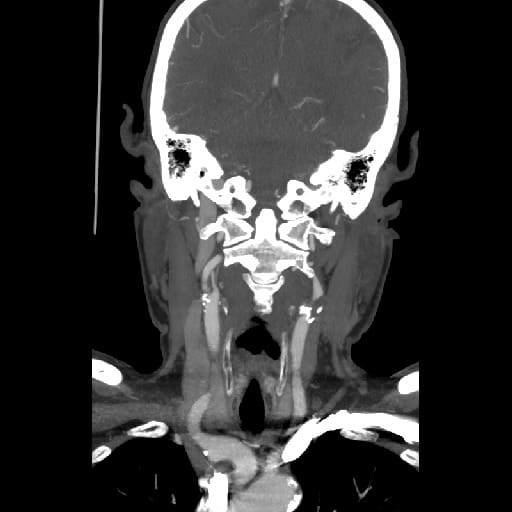
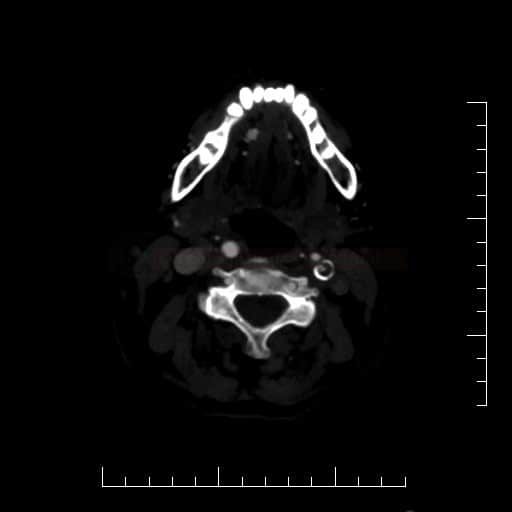
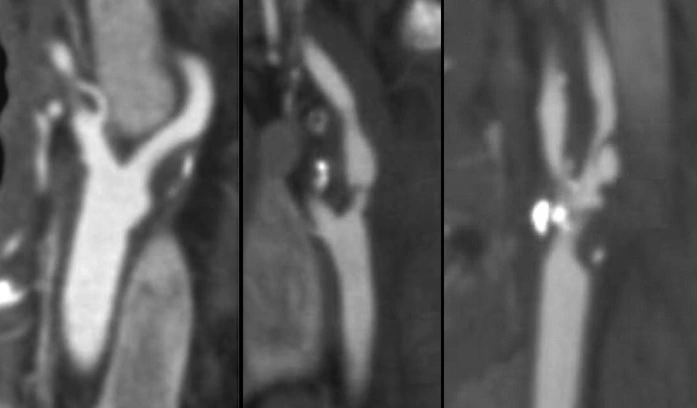
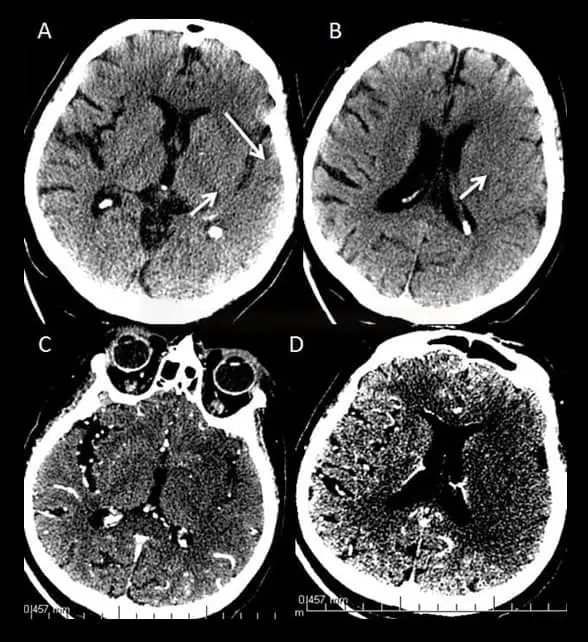
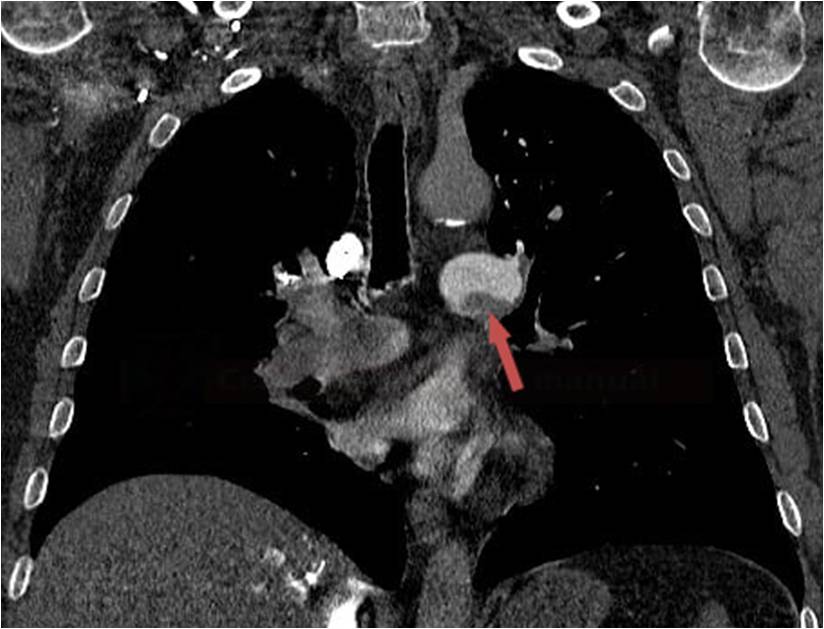
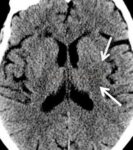
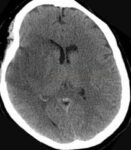
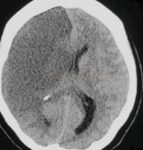
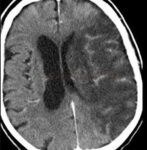
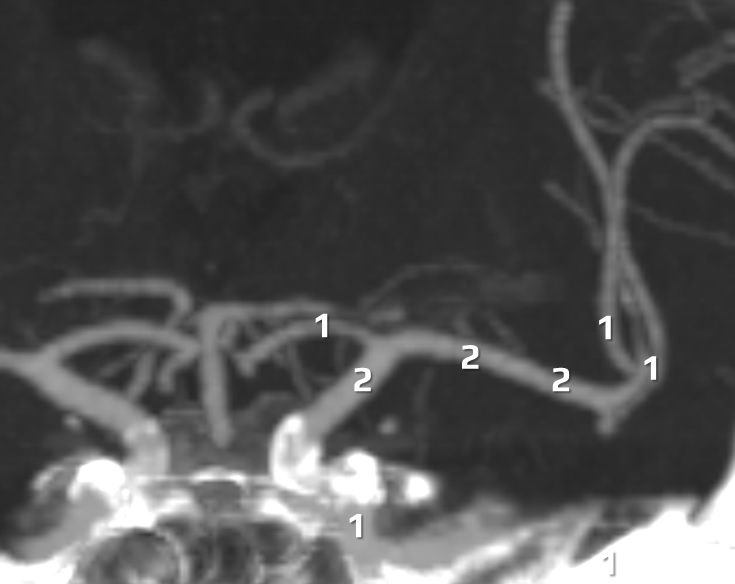
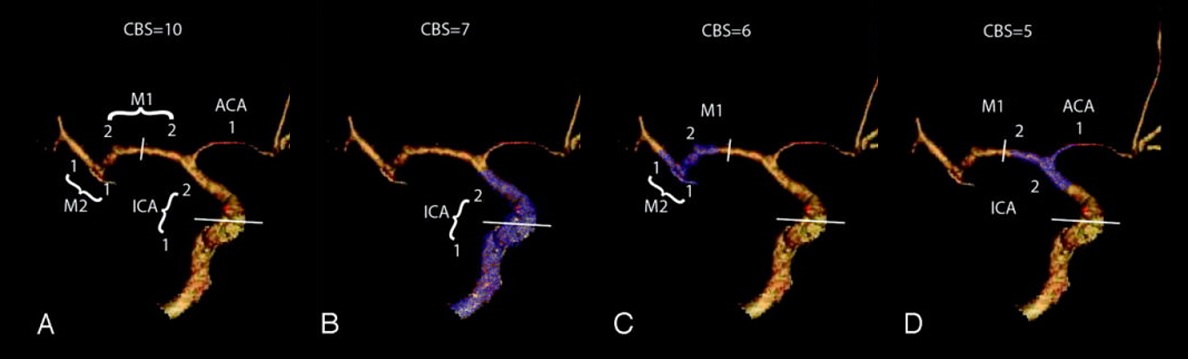
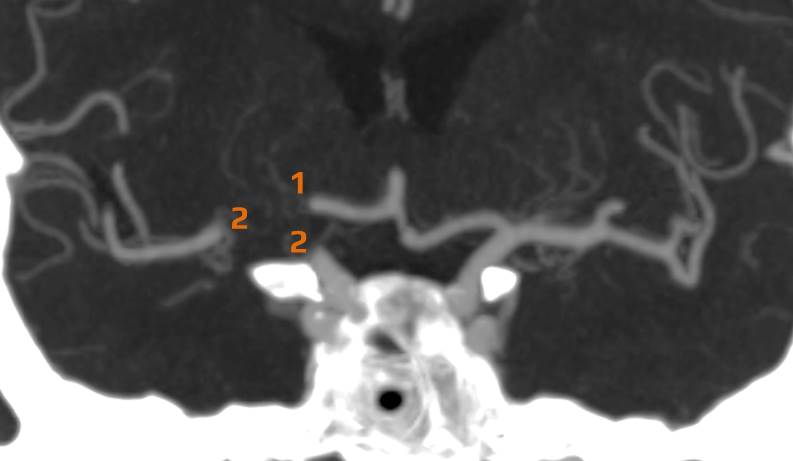
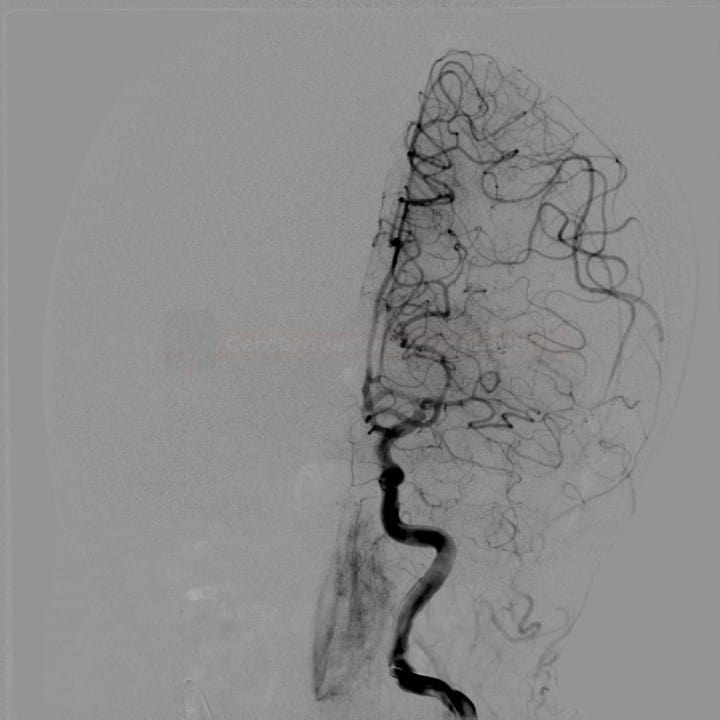
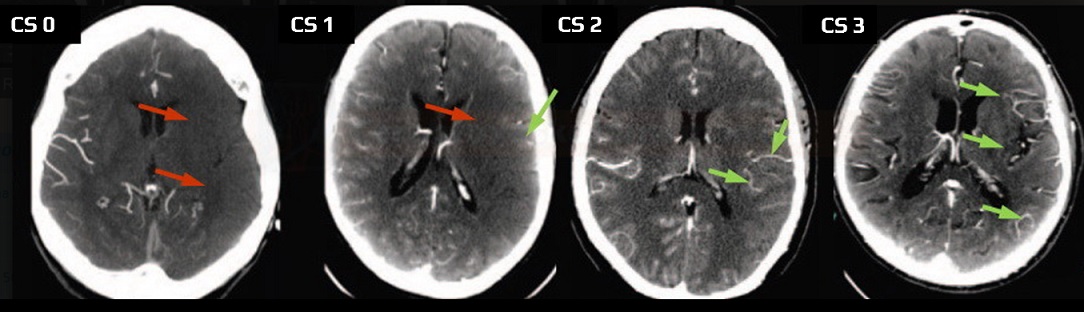
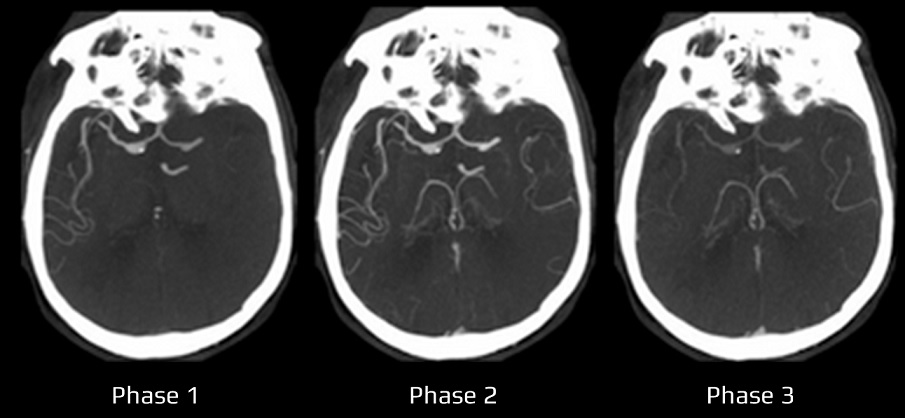
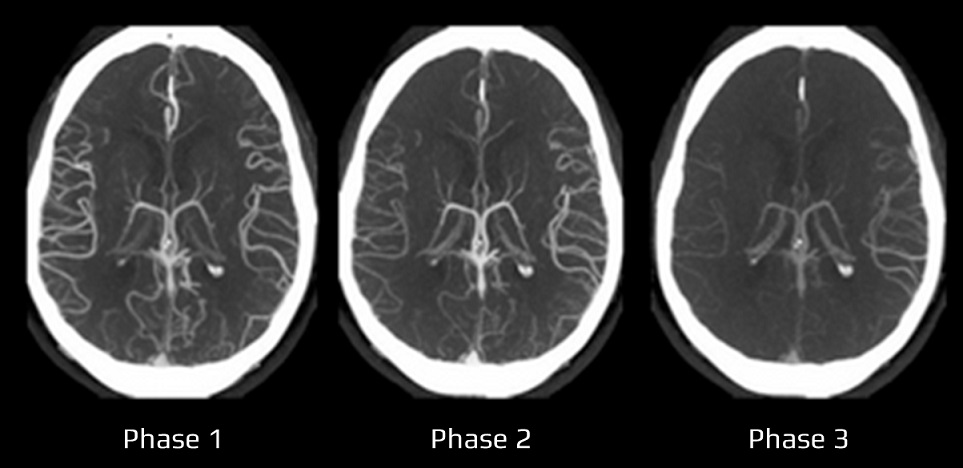
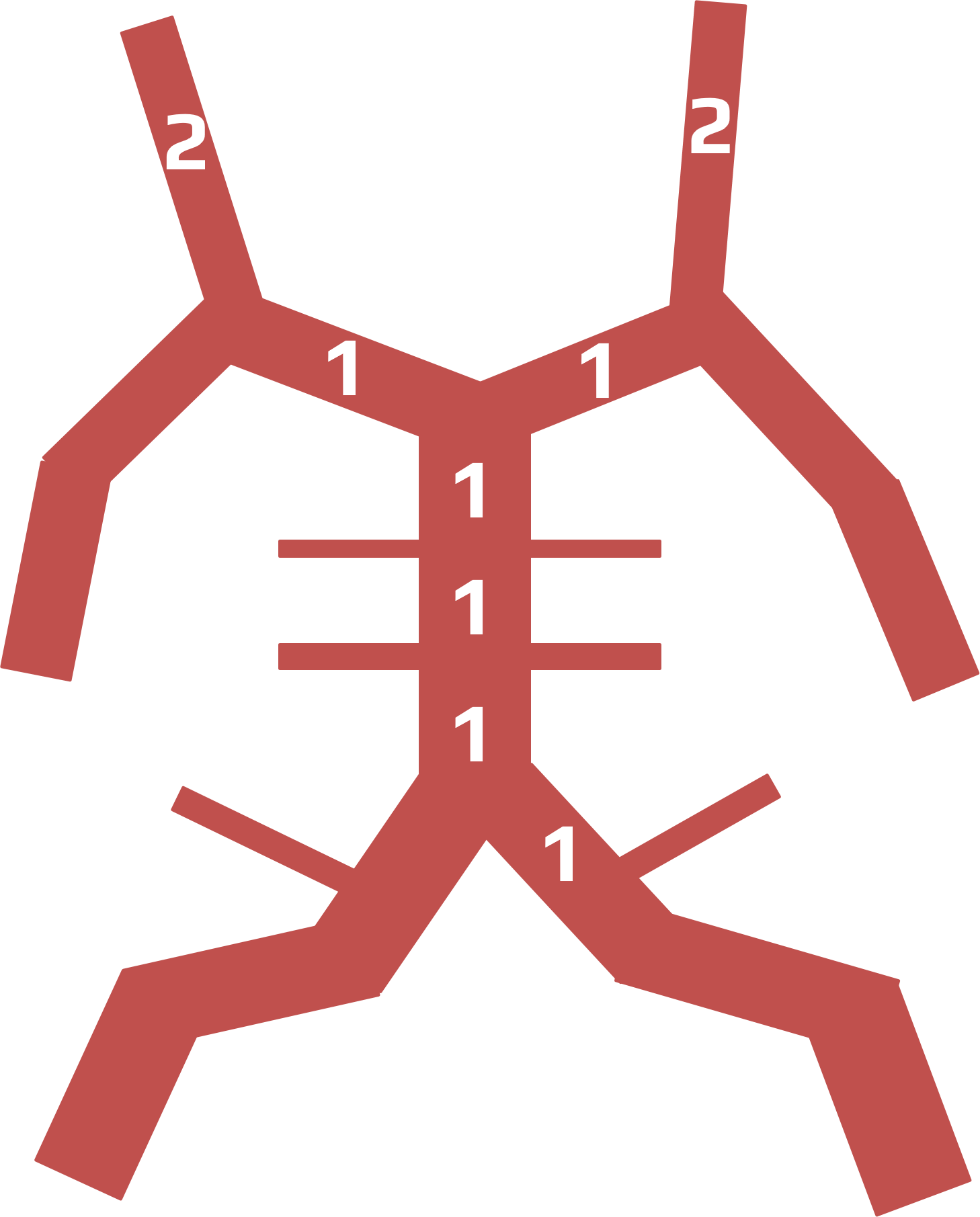
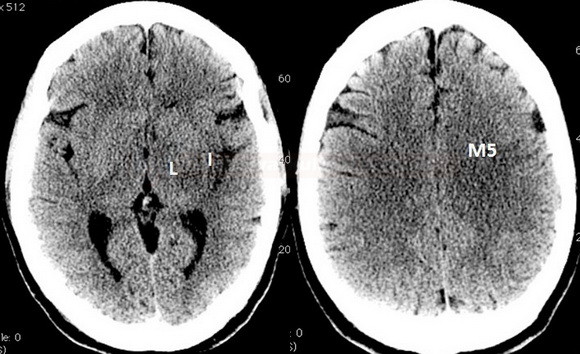
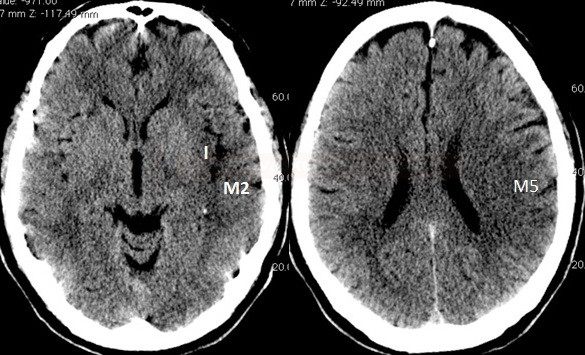
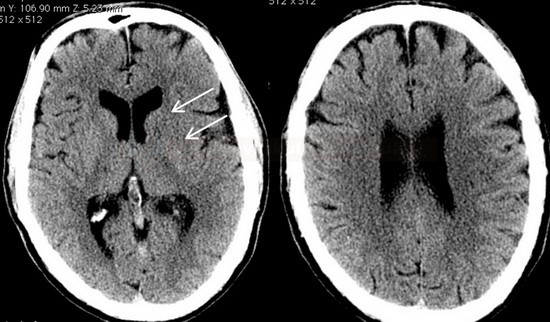
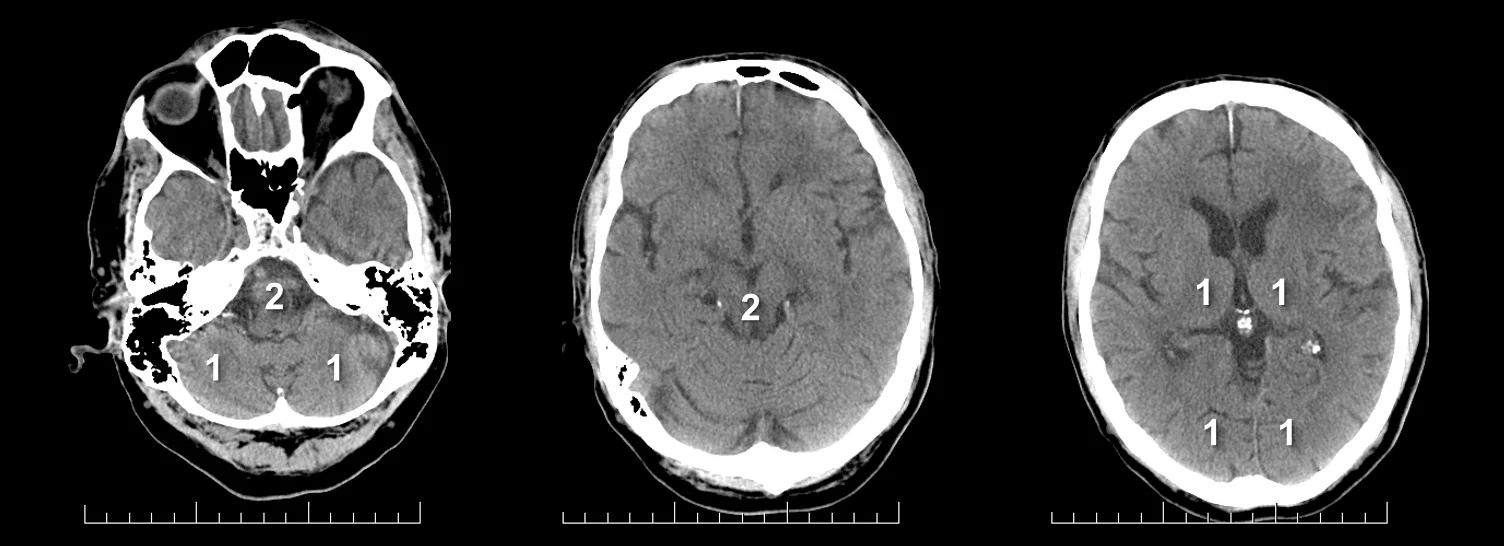
- Clot Burden Score (CBS) is a semiquantitative, CTA-based score that defines the extent of thrombosis in the anterior circulation
- 10 points are assigned for a normal CTA
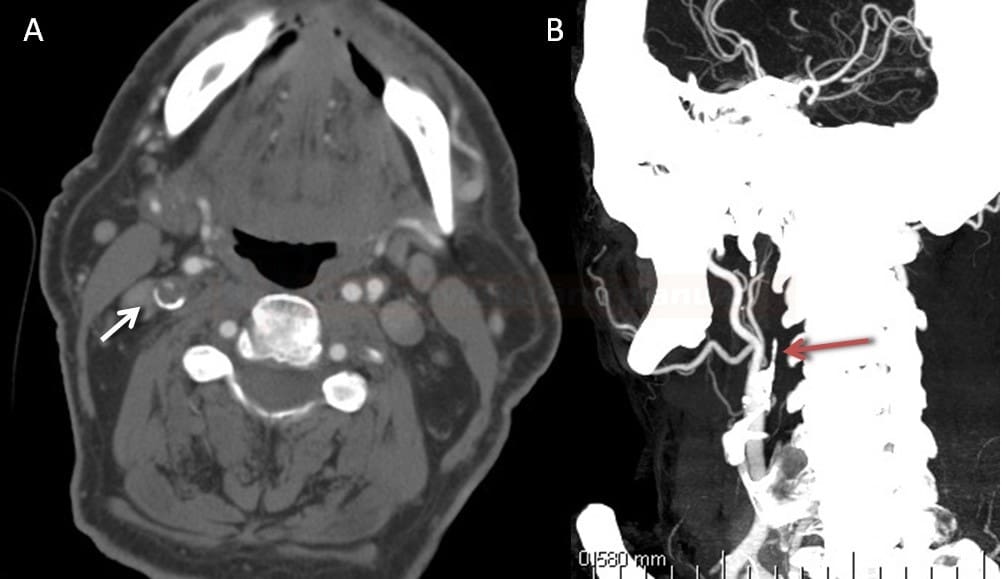
- 2 points are subtracted for thrombus in the proximal M1, distal M1, or supraclinoid ICA portion
- 1 point is subtracted for thrombus in M2 branches, A1, and/or infraclinoid ICA portion
- 2 points are subtracted for thrombus in the proximal M1, distal M1, or supraclinoid ICA portion
- the recanalization rate is higher with intravenous tPA in patients with a CBS > 6 [Demchuk, 2009]
- a lower CBS is associated with:
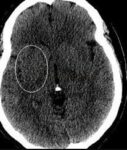
- lower ASPECTS [Puetz, 2008]
- higher rates of parenchymal hematoma [Puetz, 2008]
- decreased odds of favorable functional outcome
- odds ratio 0.09 for CBS ≤ 5, 0.22 for CBS 6-7, and 0.48 for CBS 8-9 [Puetz, 2008]
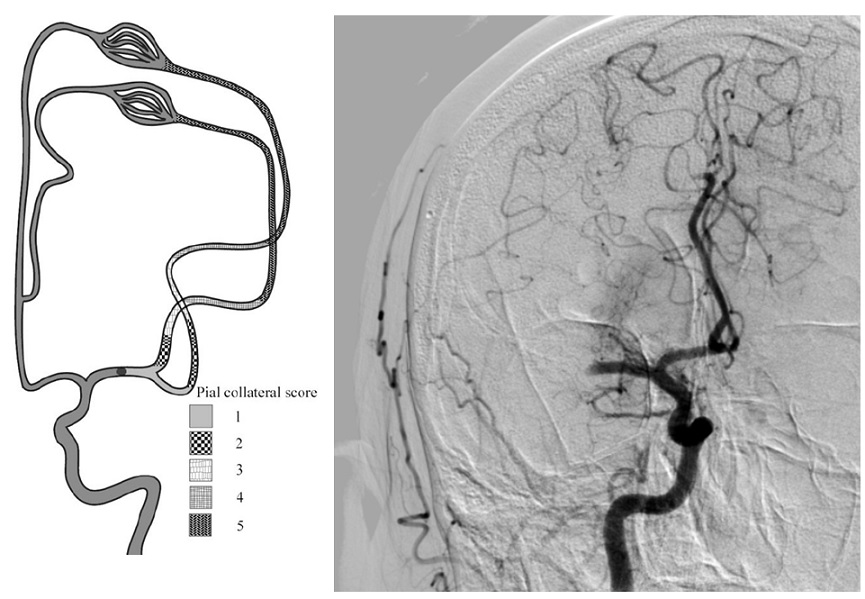
- the association between the CBS and functional outcome varies for different collateral scores (Derraz, 2021)
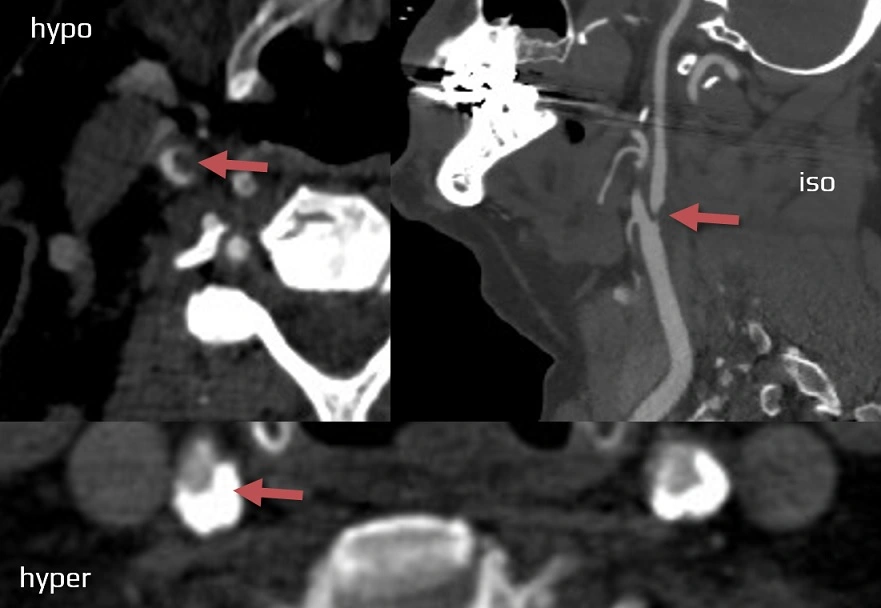
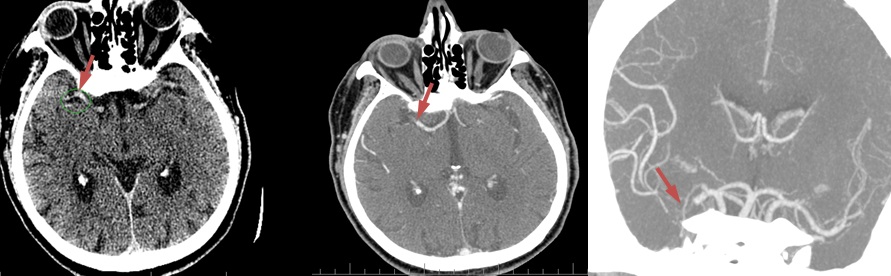
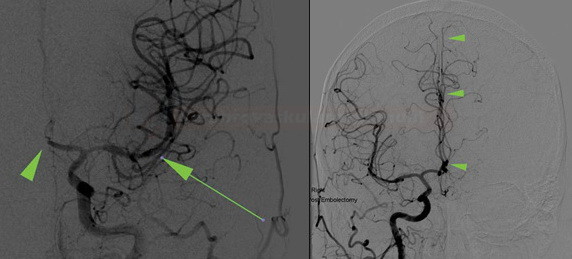
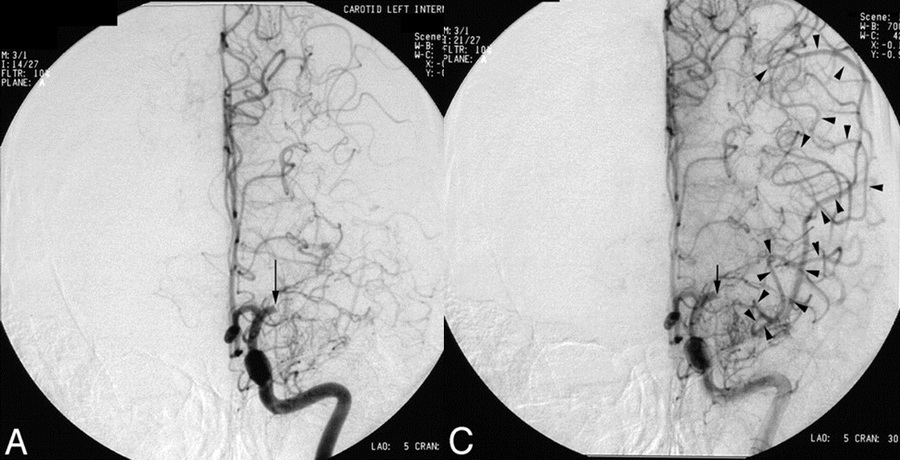
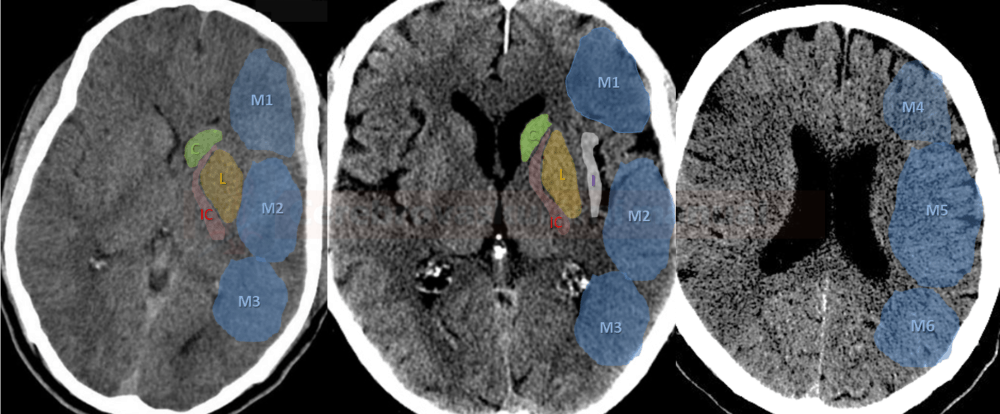
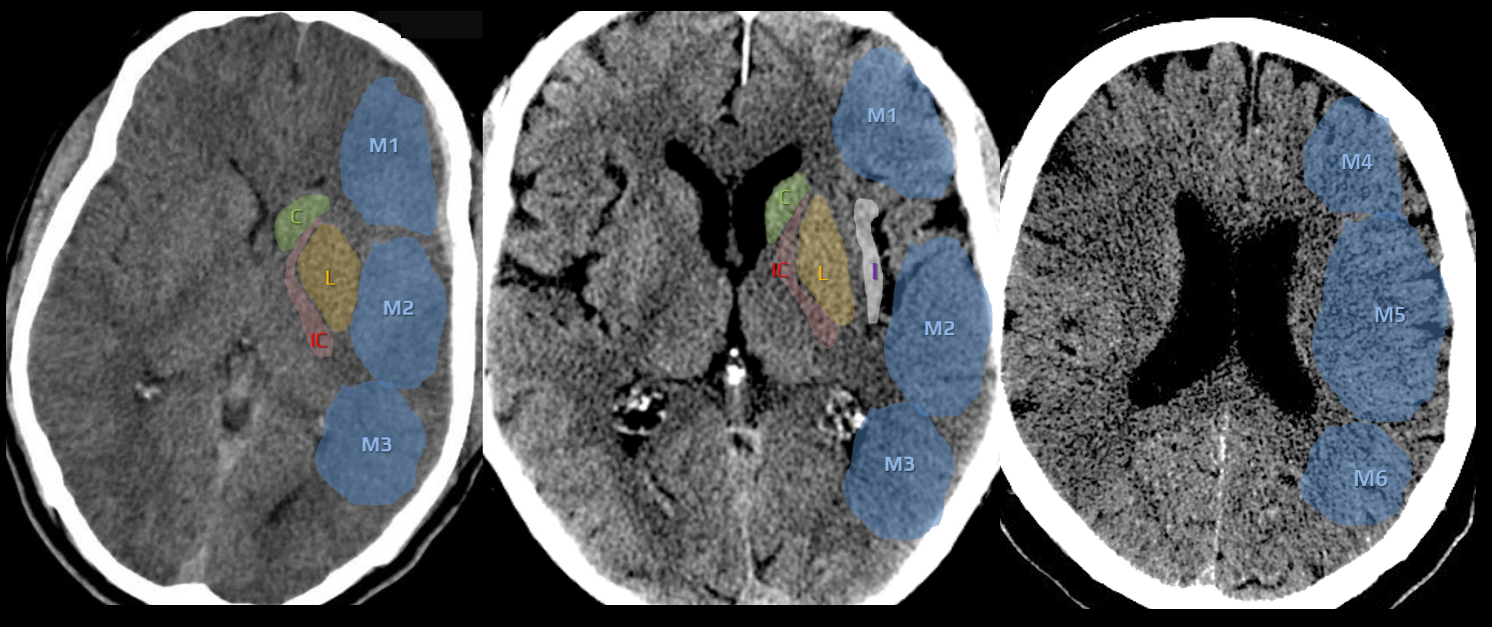
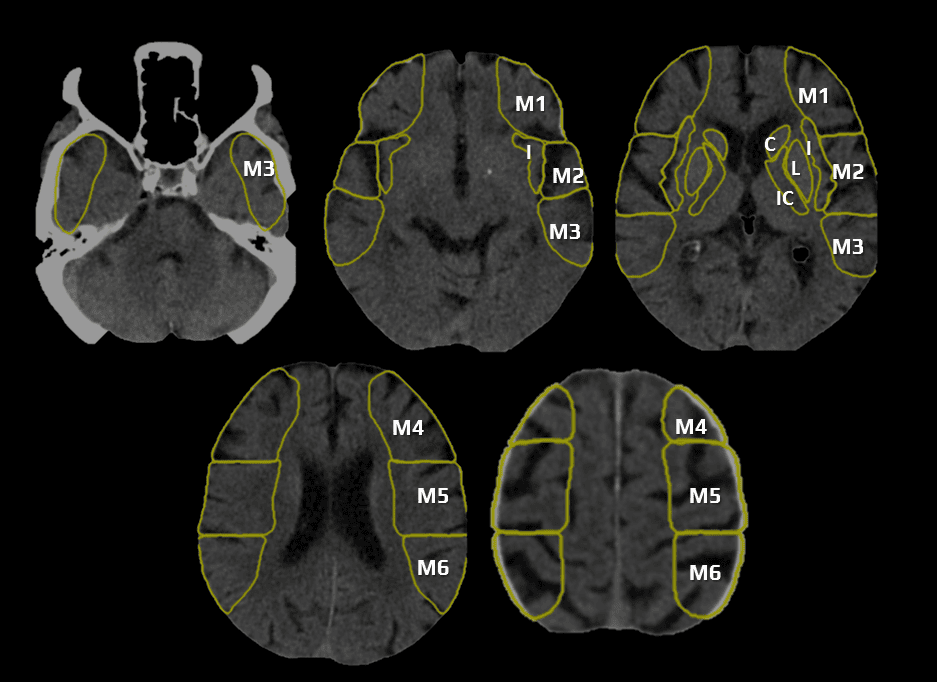
- CBS can also be assessed on FLAIR, MRA, or MR-GRE (T2*CBS)
[Derraz, 2019]
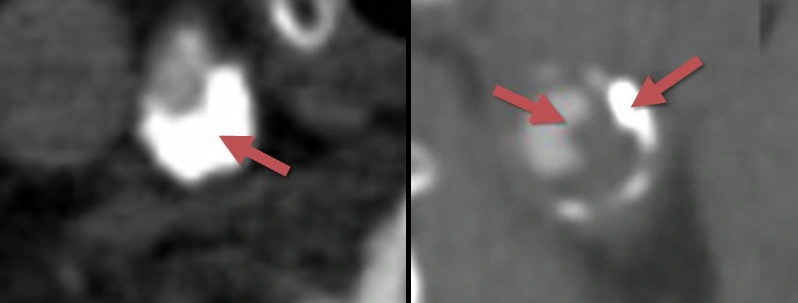
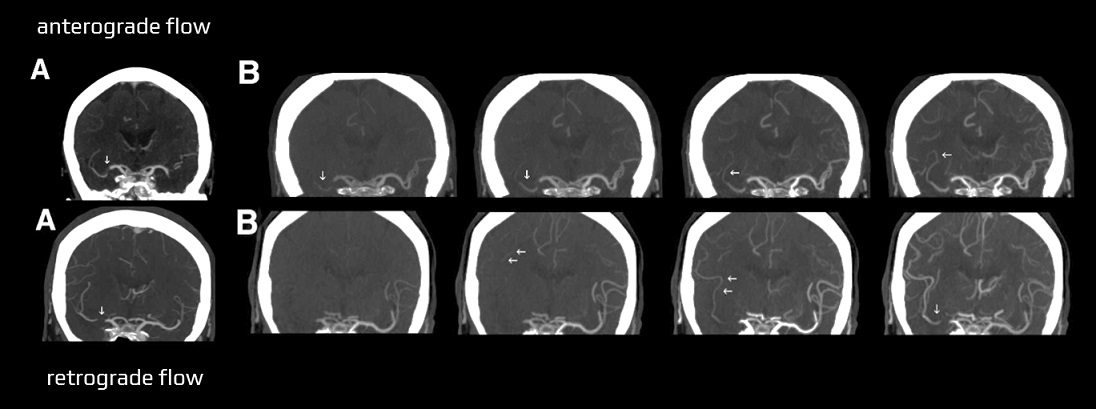
- susceptibility vessel sign (SVS)
- diameter and length (thrombus length tends to be smaller in those with early reperfusion)
- S-shaped or A-shaped
- clot intensity on FLAIR
- high FLAIR clot intensity may predict successful reperfusion (Fujimoto, 2015)
- susceptibility vessel sign (SVS)
| infraclinoid portion of ICA | – 1 |
| supraclinoid portion of ICA |
– 2 |
| proximal M1 segment |
– 2 |
| distal M1 segment |
– 2 |
| M2 branch |
– 1 |
| M2 branch |
– 1 |
| ACA | – 1 |

![pc-aspects_prognosis - Puetz pc-ASPECT score predicts prognosis [Puetz, 2009]](https://www.stroke-manual.com/wp-content/uploads/2021/03/pc-aspects_prognosis-Puetz.jpg)